| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592824 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 9 Pages |
•Non-stoichiometric complexes (NIC) were formed between cationic starches and 4-sulfophthalic acid.•Formation of NIC was confirmed by equilibrium adsorption, FT-IR and luminescence spectroscopy experiments.•Amphoteric properties of NIC determined the better flocculation efficiency.
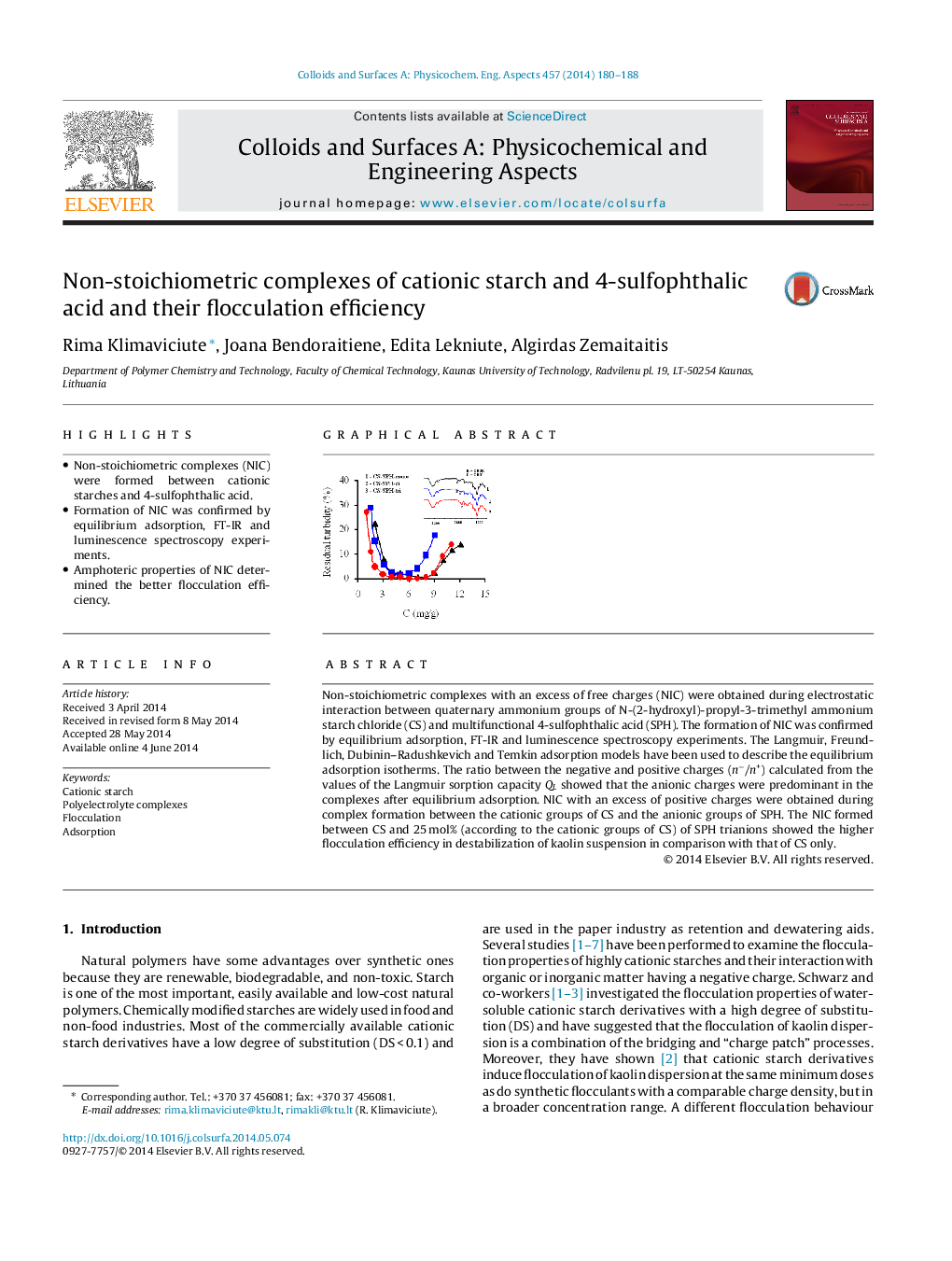
Non-stoichiometric complexes with an excess of free charges (NIC) were obtained during electrostatic interaction between quaternary ammonium groups of N-(2-hydroxyl)-propyl-3-trimethyl ammonium starch chloride (CS) and multifunctional 4-sulfophthalic acid (SPH). The formation of NIC was confirmed by equilibrium adsorption, FT-IR and luminescence spectroscopy experiments. The Langmuir, Freundlich, Dubinin–Radushkevich and Temkin adsorption models have been used to describe the equilibrium adsorption isotherms. The ratio between the negative and positive charges (n−/n+) calculated from the values of the Langmuir sorption capacity QL showed that the anionic charges were predominant in the complexes after equilibrium adsorption. NIC with an excess of positive charges were obtained during complex formation between the cationic groups of CS and the anionic groups of SPH. The NIC formed between CS and 25 mol% (according to the cationic groups of CS) of SPH trianions showed the higher flocculation efficiency in destabilization of kaolin suspension in comparison with that of CS only.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide