| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592948 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 8 Pages |
•Three N-oleoyl amino acid based surfactants were synthesized.•The surface activities and aggregation behaviours were investigated.•The micellar size and aggregation number of the surfactants were determined.
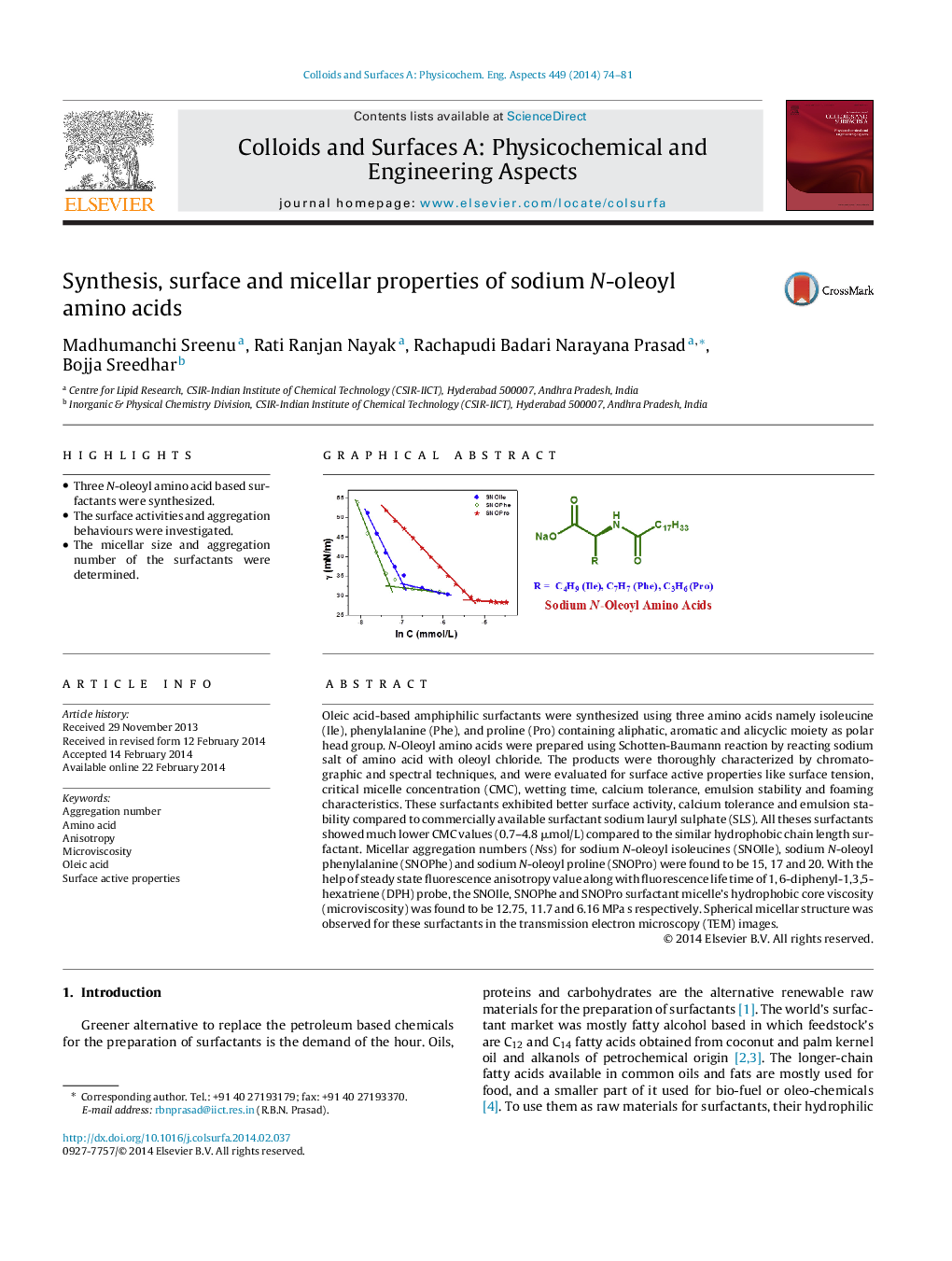
Oleic acid-based amphiphilic surfactants were synthesized using three amino acids namely isoleucine (Ile), phenylalanine (Phe), and proline (Pro) containing aliphatic, aromatic and alicyclic moiety as polar head group. N-Oleoyl amino acids were prepared using Schotten-Baumann reaction by reacting sodium salt of amino acid with oleoyl chloride. The products were thoroughly characterized by chromatographic and spectral techniques, and were evaluated for surface active properties like surface tension, critical micelle concentration (CMC), wetting time, calcium tolerance, emulsion stability and foaming characteristics. These surfactants exhibited better surface activity, calcium tolerance and emulsion stability compared to commercially available surfactant sodium lauryl sulphate (SLS). All theses surfactants showed much lower CMC values (0.7–4.8 μmol/L) compared to the similar hydrophobic chain length surfactant. Micellar aggregation numbers (Nss) for sodium N-oleoyl isoleucines (SNOIle), sodium N-oleoyl phenylalanine (SNOPhe) and sodium N-oleoyl proline (SNOPro) were found to be 15, 17 and 20. With the help of steady state fluorescence anisotropy value along with fluorescence life time of 1, 6-diphenyl-1,3,5-hexatriene (DPH) probe, the SNOIle, SNOPhe and SNOPro surfactant micelle's hydrophobic core viscosity (microviscosity) was found to be 12.75, 11.7 and 6.16 MPa s respectively. Spherical micellar structure was observed for these surfactants in the transmission electron microscopy (TEM) images.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide