| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 592960 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2014 | 11 Pages |
•Emulsification proceeds in turbulent-inertial regime at low o/w interfacial tensions.•Wide range of oils (triglycerides, silicons and alkanes) and small emulsifiers studied.•Complex interplay of process variables during emulsification process.•Droplet coalescence effects can be removed by re-circulation.•High molar-volume oils like triglycerides produce stable emulsions.
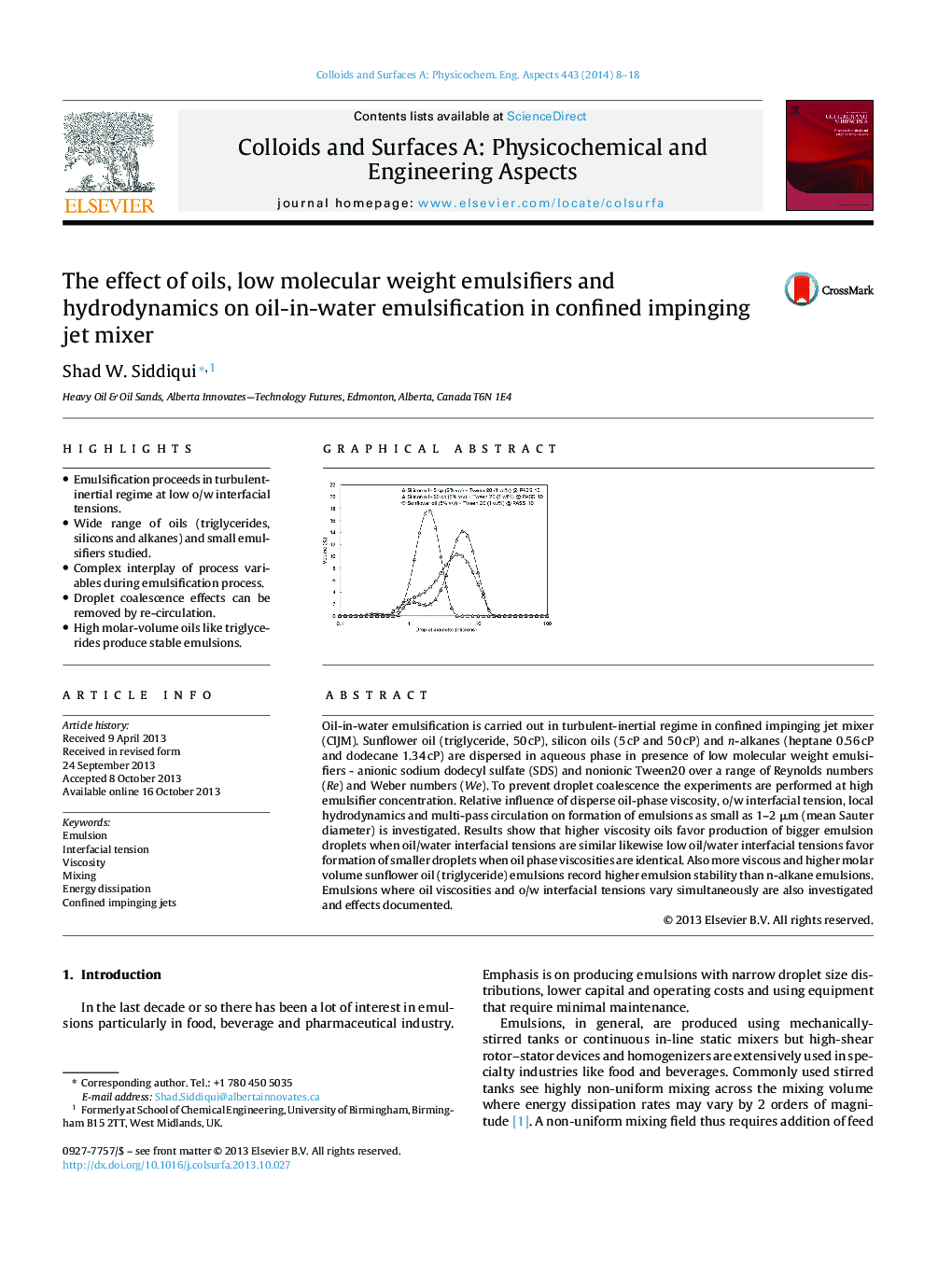
Oil-in-water emulsification is carried out in turbulent-inertial regime in confined impinging jet mixer (CIJM). Sunflower oil (triglyceride, 50 cP), silicon oils (5 cP and 50 cP) and n-alkanes (heptane 0.56 cP and dodecane 1.34 cP) are dispersed in aqueous phase in presence of low molecular weight emulsifiers - anionic sodium dodecyl sulfate (SDS) and nonionic Tween20 over a range of Reynolds numbers (Re) and Weber numbers (We). To prevent droplet coalescence the experiments are performed at high emulsifier concentration. Relative influence of disperse oil-phase viscosity, o/w interfacial tension, local hydrodynamics and multi-pass circulation on formation of emulsions as small as 1–2 μm (mean Sauter diameter) is investigated. Results show that higher viscosity oils favor production of bigger emulsion droplets when oil/water interfacial tensions are similar likewise low oil/water interfacial tensions favor formation of smaller droplets when oil phase viscosities are identical. Also more viscous and higher molar volume sunflower oil (triglyceride) emulsions record higher emulsion stability than n-alkane emulsions. Emulsions where oil viscosities and o/w interfacial tensions vary simultaneously are also investigated and effects documented.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide