| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593637 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2013 | 8 Pages |
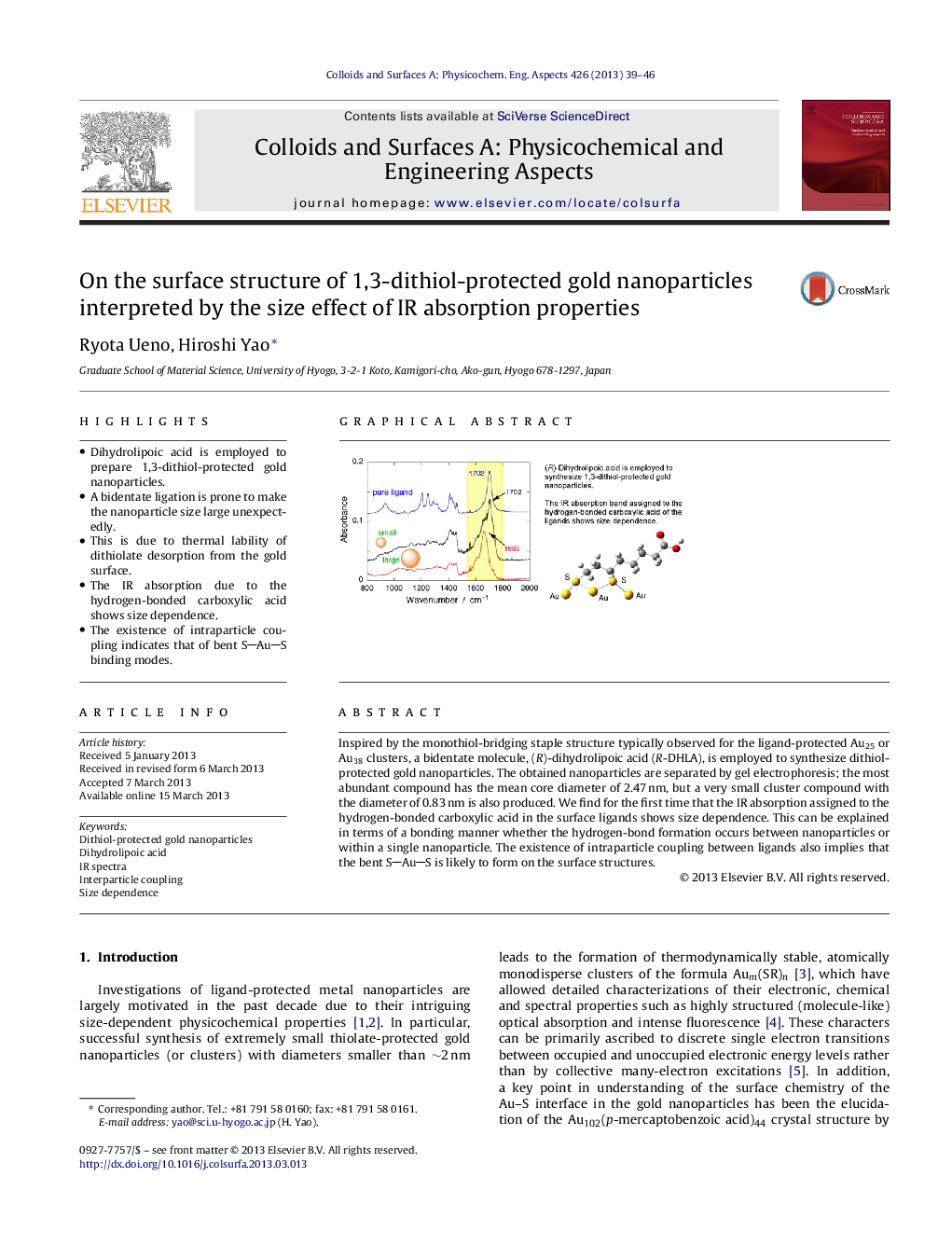
•Dihydrolipoic acid is employed to prepare 1,3-dithiol-protected gold nanoparticles.•A bidentate ligation is prone to make the nanoparticle size large unexpectedly.•This is due to thermal lability of dithiolate desorption from the gold surface.•The IR absorption due to the hydrogen-bonded carboxylic acid shows size dependence.•The existence of intraparticle coupling indicates that of bent SAuS binding modes.
Inspired by the monothiol-bridging staple structure typically observed for the ligand-protected Au25 or Au38 clusters, a bidentate molecule, (R)-dihydrolipoic acid (R-DHLA), is employed to synthesize dithiol-protected gold nanoparticles. The obtained nanoparticles are separated by gel electrophoresis; the most abundant compound has the mean core diameter of 2.47 nm, but a very small cluster compound with the diameter of 0.83 nm is also produced. We find for the first time that the IR absorption assigned to the hydrogen-bonded carboxylic acid in the surface ligands shows size dependence. This can be explained in terms of a bonding manner whether the hydrogen-bond formation occurs between nanoparticles or within a single nanoparticle. The existence of intraparticle coupling between ligands also implies that the bent SAuS is likely to form on the surface structures.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide