| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593714 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2013 | 14 Pages |
•Ionic liquid-cationic surfactant interactions were studied by various techniques.•The mixed micelles were found to be generally spherical.•Most of the mixtures exhibit synergistic behavior.•Deshielding of ionic liquid protons observed at higher concentration of surfactants.
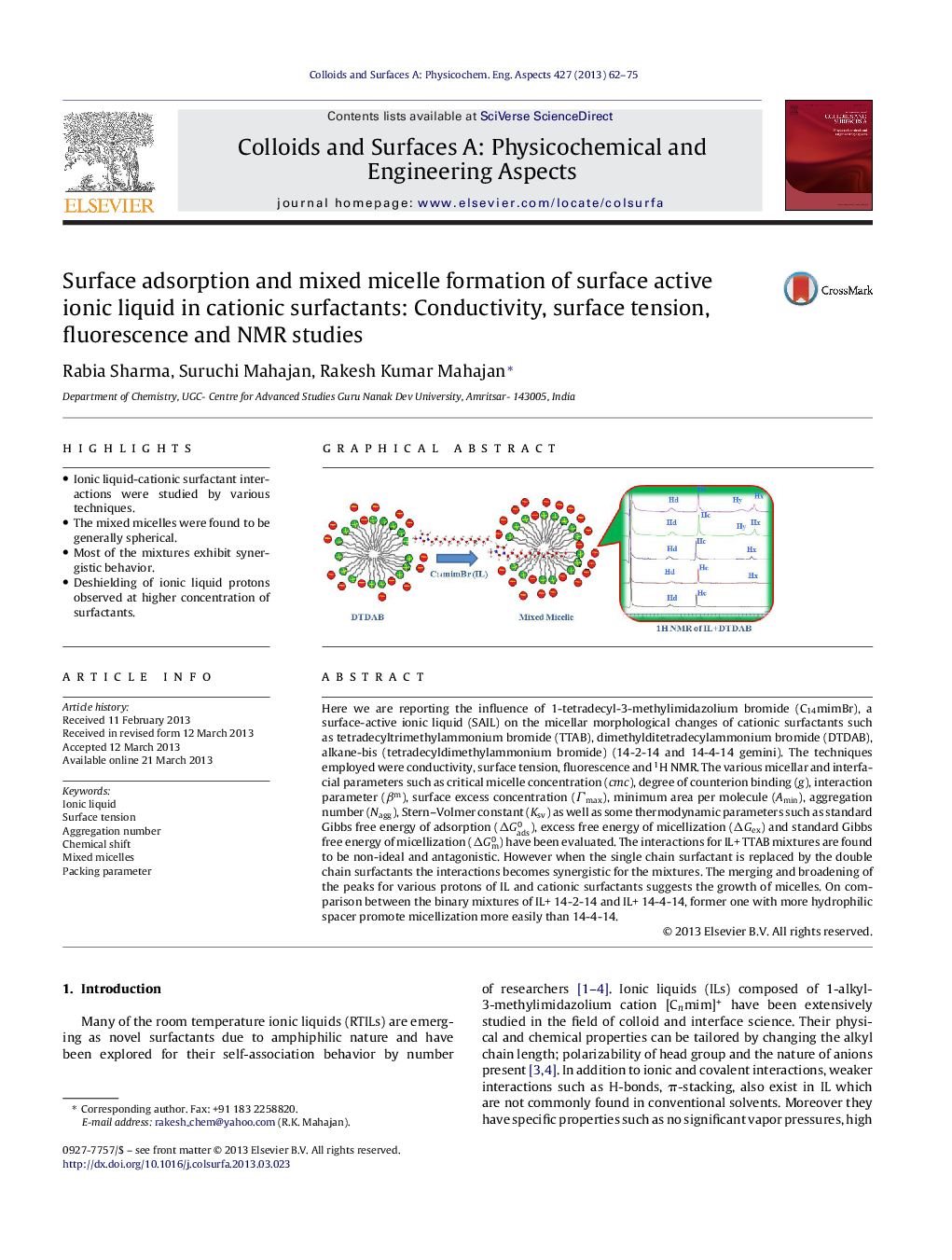
Here we are reporting the influence of 1-tetradecyl-3-methylimidazolium bromide (C14mimBr), a surface-active ionic liquid (SAIL) on the micellar morphological changes of cationic surfactants such as tetradecyltrimethylammonium bromide (TTAB), dimethylditetradecylammonium bromide (DTDAB), alkane-bis (tetradecyldimethylammonium bromide) (14-2-14 and 14-4-14 gemini). The techniques employed were conductivity, surface tension, fluorescence and 1H NMR. The various micellar and interfacial parameters such as critical micelle concentration (cmc), degree of counterion binding (g), interaction parameter (βm), surface excess concentration (Гmax), minimum area per molecule (Amin), aggregation number (Nagg), Stern–Volmer constant (Ksv) as well as some thermodynamic parameters such as standard Gibbs free energy of adsorption (ΔGads0), excess free energy of micellization (ΔGex) and standard Gibbs free energy of micellization (ΔGm0) have been evaluated. The interactions for IL+ TTAB mixtures are found to be non-ideal and antagonistic. However when the single chain surfactant is replaced by the double chain surfactants the interactions becomes synergistic for the mixtures. The merging and broadening of the peaks for various protons of IL and cationic surfactants suggests the growth of micelles. On comparison between the binary mixtures of IL+ 14-2-14 and IL+ 14-4-14, former one with more hydrophilic spacer promote micellization more easily than 14-4-14.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide