| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593777 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2013 | 9 Pages |
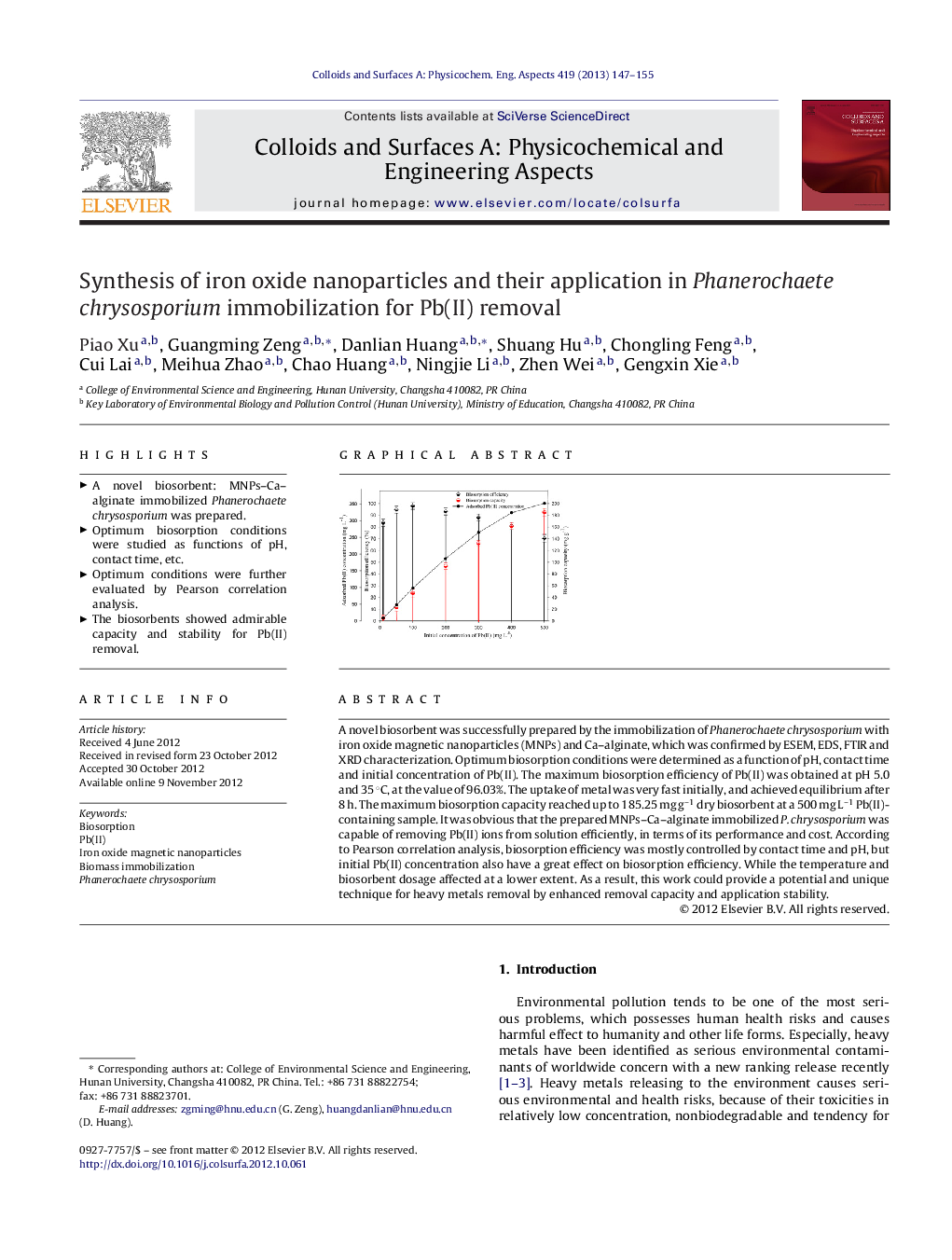
A novel biosorbent was successfully prepared by the immobilization of Phanerochaete chrysosporium with iron oxide magnetic nanoparticles (MNPs) and Ca-alginate, which was confirmed by ESEM, EDS, FTIR and XRD characterization. Optimum biosorption conditions were determined as a function of pH, contact time and initial concentration of Pb(II). The maximum biosorption efficiency of Pb(II) was obtained at pH 5.0 and 35 °C, at the value of 96.03%. The uptake of metal was very fast initially, and achieved equilibrium after 8 h. The maximum biosorption capacity reached up to 185.25 mg g−1 dry biosorbent at a 500 mg L−1 Pb(II)-containing sample. It was obvious that the prepared MNPs-Ca-alginate immobilized P. chrysosporium was capable of removing Pb(II) ions from solution efficiently, in terms of its performance and cost. According to Pearson correlation analysis, biosorption efficiency was mostly controlled by contact time and pH, but initial Pb(II) concentration also have a great effect on biosorption efficiency. While the temperature and biosorbent dosage affected at a lower extent. As a result, this work could provide a potential and unique technique for heavy metals removal by enhanced removal capacity and application stability.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► A novel biosorbent: MNPs-Ca-alginate immobilized P. chrysosporium was prepared. ► Optimum biosorption conditions were studied as functions of pH, contact time, et al. ► Optimum conditions were further evaluated by Pearson correlation analysis. ► The biosorbents showed admirable capacity and stability for Pb(II) removal.