| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593798 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2013 | 6 Pages |
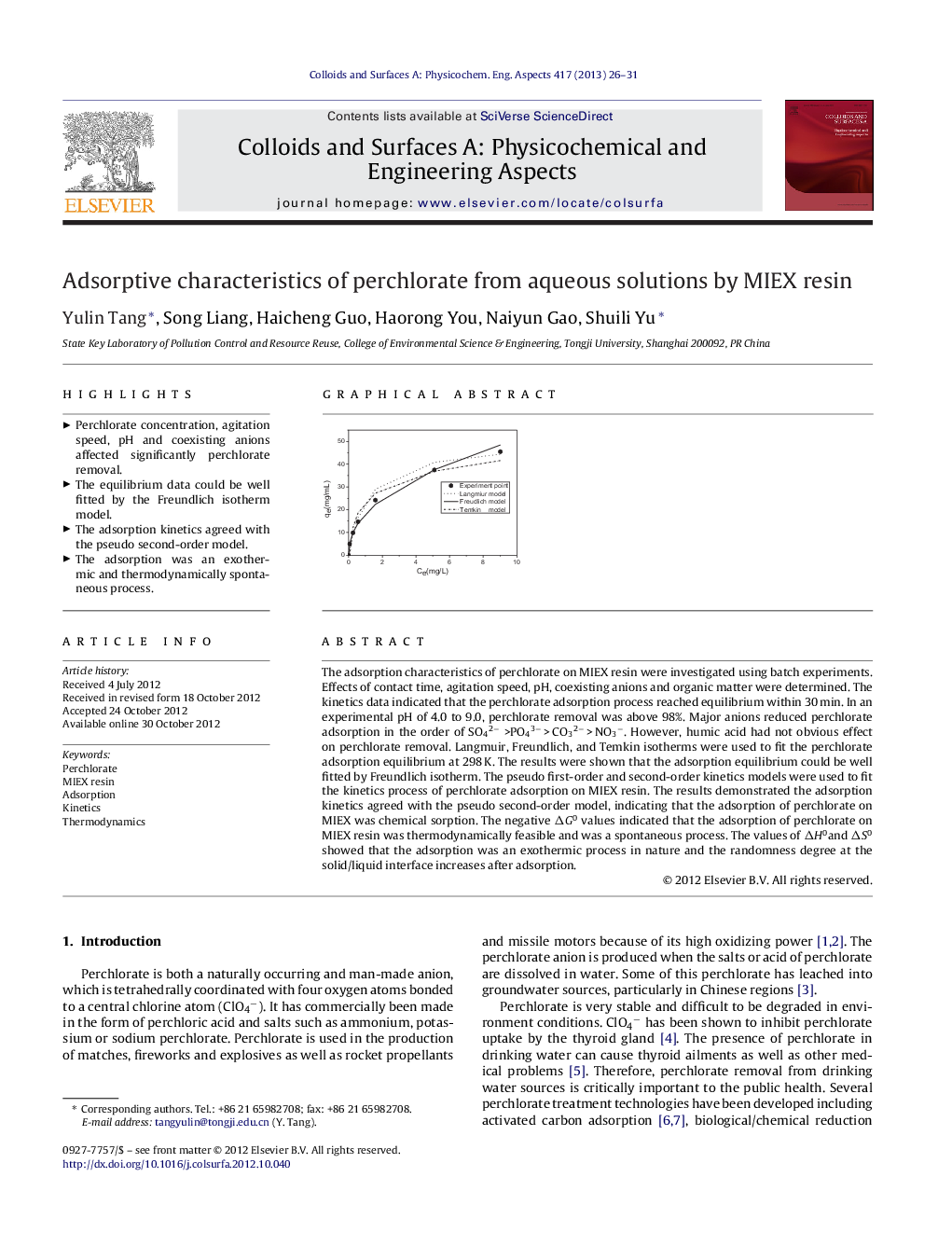
The adsorption characteristics of perchlorate on MIEX resin were investigated using batch experiments. Effects of contact time, agitation speed, pH, coexisting anions and organic matter were determined. The kinetics data indicated that the perchlorate adsorption process reached equilibrium within 30 min. In an experimental pH of 4.0 to 9.0, perchlorate removal was above 98%. Major anions reduced perchlorate adsorption in the order of SO42− >PO43− > CO32− > NO3−. However, humic acid had not obvious effect on perchlorate removal. Langmuir, Freundlich, and Temkin isotherms were used to fit the perchlorate adsorption equilibrium at 298 K. The results were shown that the adsorption equilibrium could be well fitted by Freundlich isotherm. The pseudo first-order and second-order kinetics models were used to fit the kinetics process of perchlorate adsorption on MIEX resin. The results demonstrated the adsorption kinetics agreed with the pseudo second-order model, indicating that the adsorption of perchlorate on MIEX was chemical sorption. The negative ΔG0 values indicated that the adsorption of perchlorate on MIEX resin was thermodynamically feasible and was a spontaneous process. The values of ΔH0and ΔS0 showed that the adsorption was an exothermic process in nature and the randomness degree at the solid/liquid interface increases after adsorption.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Perchlorate concentration, agitation speed, pH and coexisting anions affected significantly perchlorate removal. ► The equilibrium data could be well fitted by the Freundlich isotherm model. ► The adsorption kinetics agreed with the pseudo second-order model. ► The adsorption was an exothermic and thermodynamically spontaneous process.