| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593824 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2013 | 6 Pages |
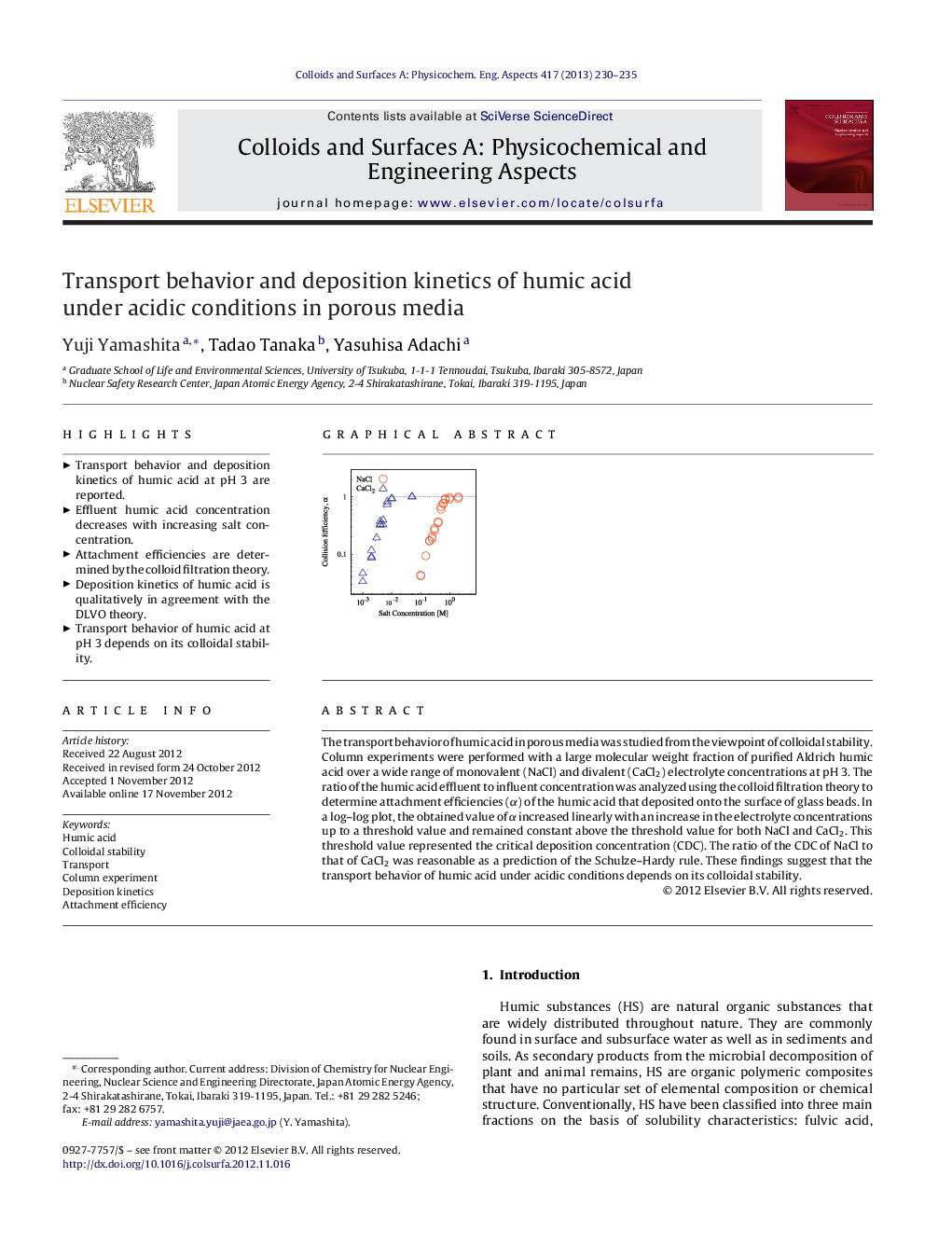
The transport behavior of humic acid in porous media was studied from the viewpoint of colloidal stability. Column experiments were performed with a large molecular weight fraction of purified Aldrich humic acid over a wide range of monovalent (NaCl) and divalent (CaCl2) electrolyte concentrations at pH 3. The ratio of the humic acid effluent to influent concentration was analyzed using the colloid filtration theory to determine attachment efficiencies (α) of the humic acid that deposited onto the surface of glass beads. In a log–log plot, the obtained value of α increased linearly with an increase in the electrolyte concentrations up to a threshold value and remained constant above the threshold value for both NaCl and CaCl2. This threshold value represented the critical deposition concentration (CDC). The ratio of the CDC of NaCl to that of CaCl2 was reasonable as a prediction of the Schulze–Hardy rule. These findings suggest that the transport behavior of humic acid under acidic conditions depends on its colloidal stability.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Transport behavior and deposition kinetics of humic acid at pH 3 are reported. ► Effluent humic acid concentration decreases with increasing salt concentration. ► Attachment efficiencies are determined by the colloid filtration theory. ► Deposition kinetics of humic acid is qualitatively in agreement with the DLVO theory. ► Transport behavior of humic acid at pH 3 depends on its colloidal stability.