| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593872 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2012 | 12 Pages |
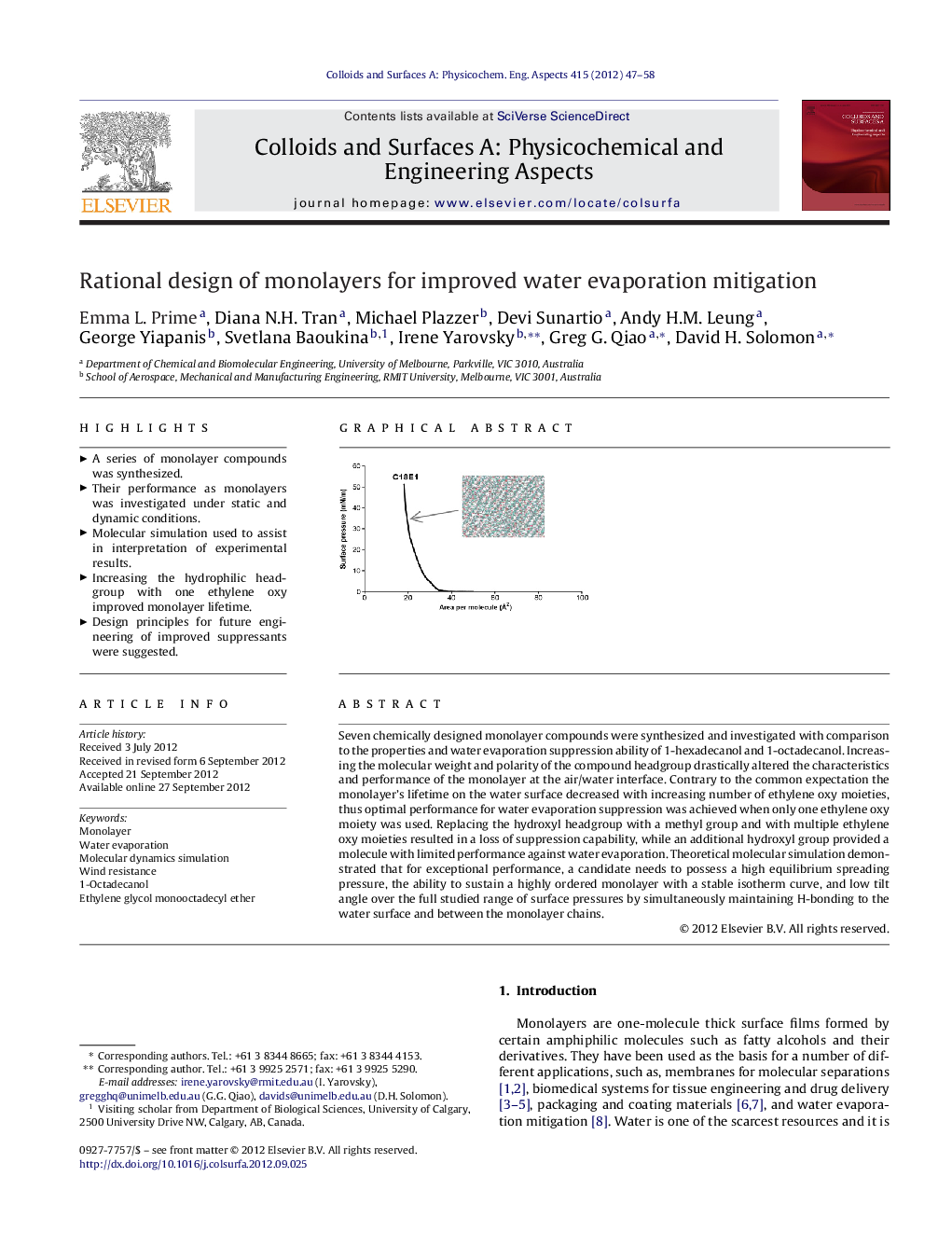
Seven chemically designed monolayer compounds were synthesized and investigated with comparison to the properties and water evaporation suppression ability of 1-hexadecanol and 1-octadecanol. Increasing the molecular weight and polarity of the compound headgroup drastically altered the characteristics and performance of the monolayer at the air/water interface. Contrary to the common expectation the monolayer's lifetime on the water surface decreased with increasing number of ethylene oxy moieties, thus optimal performance for water evaporation suppression was achieved when only one ethylene oxy moiety was used. Replacing the hydroxyl headgroup with a methyl group and with multiple ethylene oxy moieties resulted in a loss of suppression capability, while an additional hydroxyl group provided a molecule with limited performance against water evaporation. Theoretical molecular simulation demonstrated that for exceptional performance, a candidate needs to possess a high equilibrium spreading pressure, the ability to sustain a highly ordered monolayer with a stable isotherm curve, and low tilt angle over the full studied range of surface pressures by simultaneously maintaining H-bonding to the water surface and between the monolayer chains.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► A series of monolayer compounds was synthesized. ► Their performance as monolayers was investigated under static and dynamic conditions. ► Molecular simulation used to assist in interpretation of experimental results. ► Increasing the hydrophilic headgroup with one ethylene oxy improved monolayer lifetime. ► Design principles for future engineering of improved suppressants were suggested.