| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 593977 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2012 | 10 Pages |
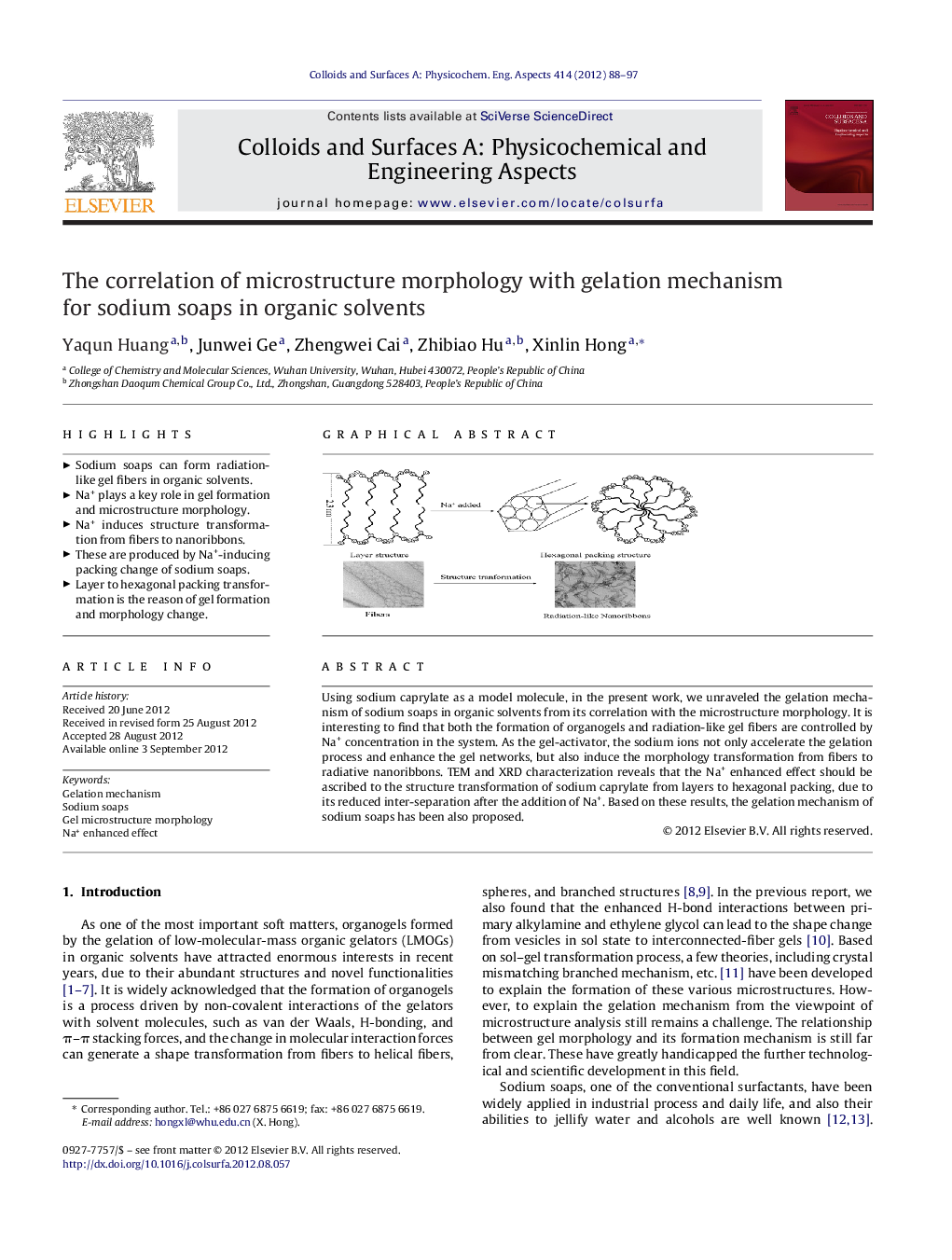
Using sodium caprylate as a model molecule, in the present work, we unraveled the gelation mechanism of sodium soaps in organic solvents from its correlation with the microstructure morphology. It is interesting to find that both the formation of organogels and radiation-like gel fibers are controlled by Na+ concentration in the system. As the gel-activator, the sodium ions not only accelerate the gelation process and enhance the gel networks, but also induce the morphology transformation from fibers to radiative nanoribbons. TEM and XRD characterization reveals that the Na+ enhanced effect should be ascribed to the structure transformation of sodium caprylate from layers to hexagonal packing, due to its reduced inter-separation after the addition of Na+. Based on these results, the gelation mechanism of sodium soaps has been also proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Sodium soaps can form radiation-like gel fibers in organic solvents. ► Na+ plays a key role in gel formation and microstructure morphology. ► Na+ induces structure transformation from fibers to nanoribbons. ► These are produced by Na+-inducing packing change of sodium soaps. ► Layer to hexagonal packing transformation is the reason of gel formation and morphology change.