| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 594001 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2012 | 7 Pages |
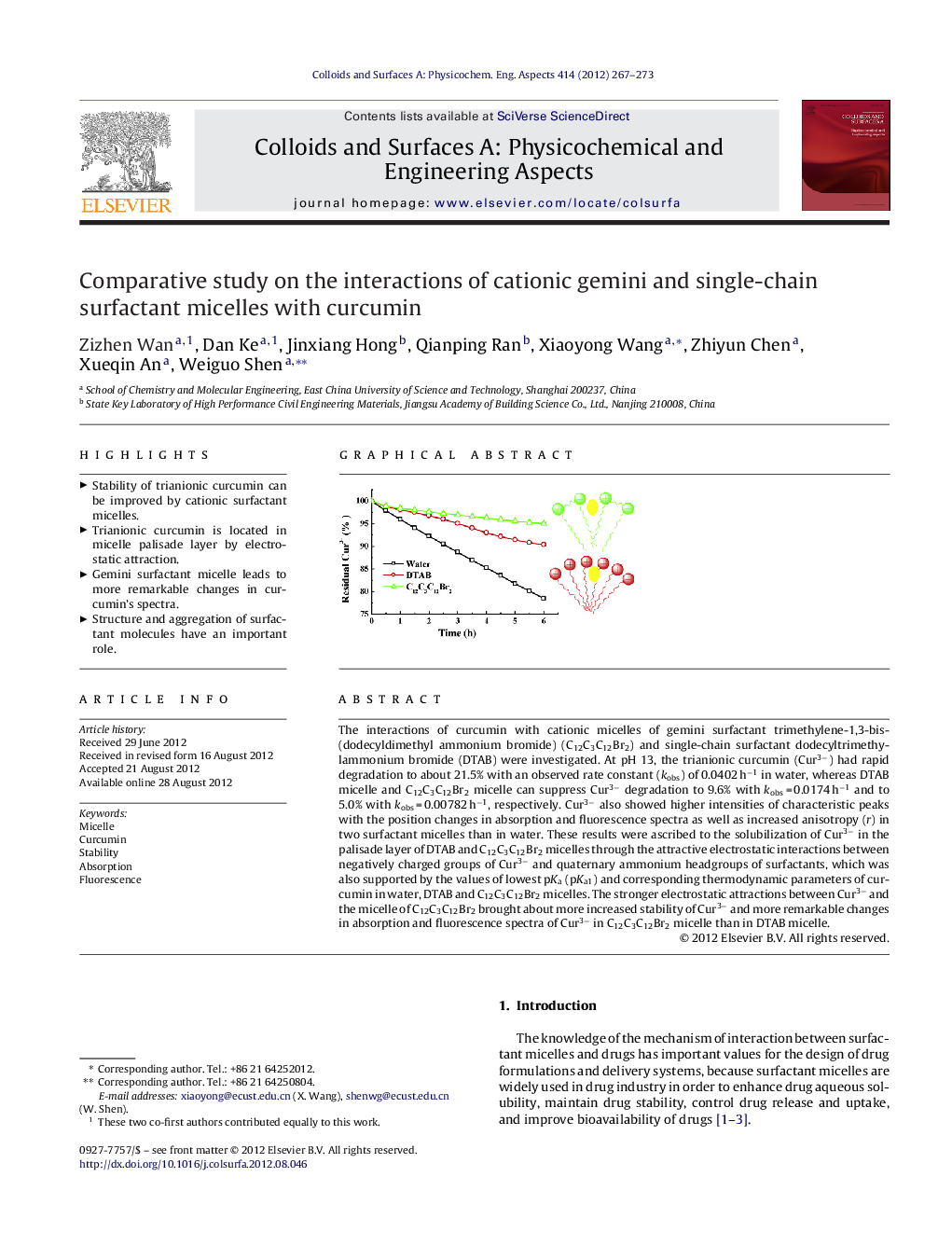
The interactions of curcumin with cationic micelles of gemini surfactant trimethylene-1,3-bis-(dodecyldimethyl ammonium bromide) (C12C3C12Br2) and single-chain surfactant dodecyltrimethylammonium bromide (DTAB) were investigated. At pH 13, the trianionic curcumin (Cur3−) had rapid degradation to about 21.5% with an observed rate constant (kobs) of 0.0402 h−1 in water, whereas DTAB micelle and C12C3C12Br2 micelle can suppress Cur3− degradation to 9.6% with kobs = 0.0174 h−1 and to 5.0% with kobs = 0.00782 h−1, respectively. Cur3− also showed higher intensities of characteristic peaks with the position changes in absorption and fluorescence spectra as well as increased anisotropy (r) in two surfactant micelles than in water. These results were ascribed to the solubilization of Cur3− in the palisade layer of DTAB and C12C3C12Br2 micelles through the attractive electrostatic interactions between negatively charged groups of Cur3− and quaternary ammonium headgroups of surfactants, which was also supported by the values of lowest pKa (pKa1) and corresponding thermodynamic parameters of curcumin in water, DTAB and C12C3C12Br2 micelles. The stronger electrostatic attractions between Cur3− and the micelle of C12C3C12Br2 brought about more increased stability of Cur3− and more remarkable changes in absorption and fluorescence spectra of Cur3− in C12C3C12Br2 micelle than in DTAB micelle.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Stability of trianionic curcumin can be improved by cationic surfactant micelles. ► Trianionic curcumin is located in micelle palisade layer by electrostatic attraction. ► Gemini surfactant micelle leads to more remarkable changes in curcumin's spectra. ► Structure and aggregation of surfactant molecules have an important role.