| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 594014 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2012 | 7 Pages |
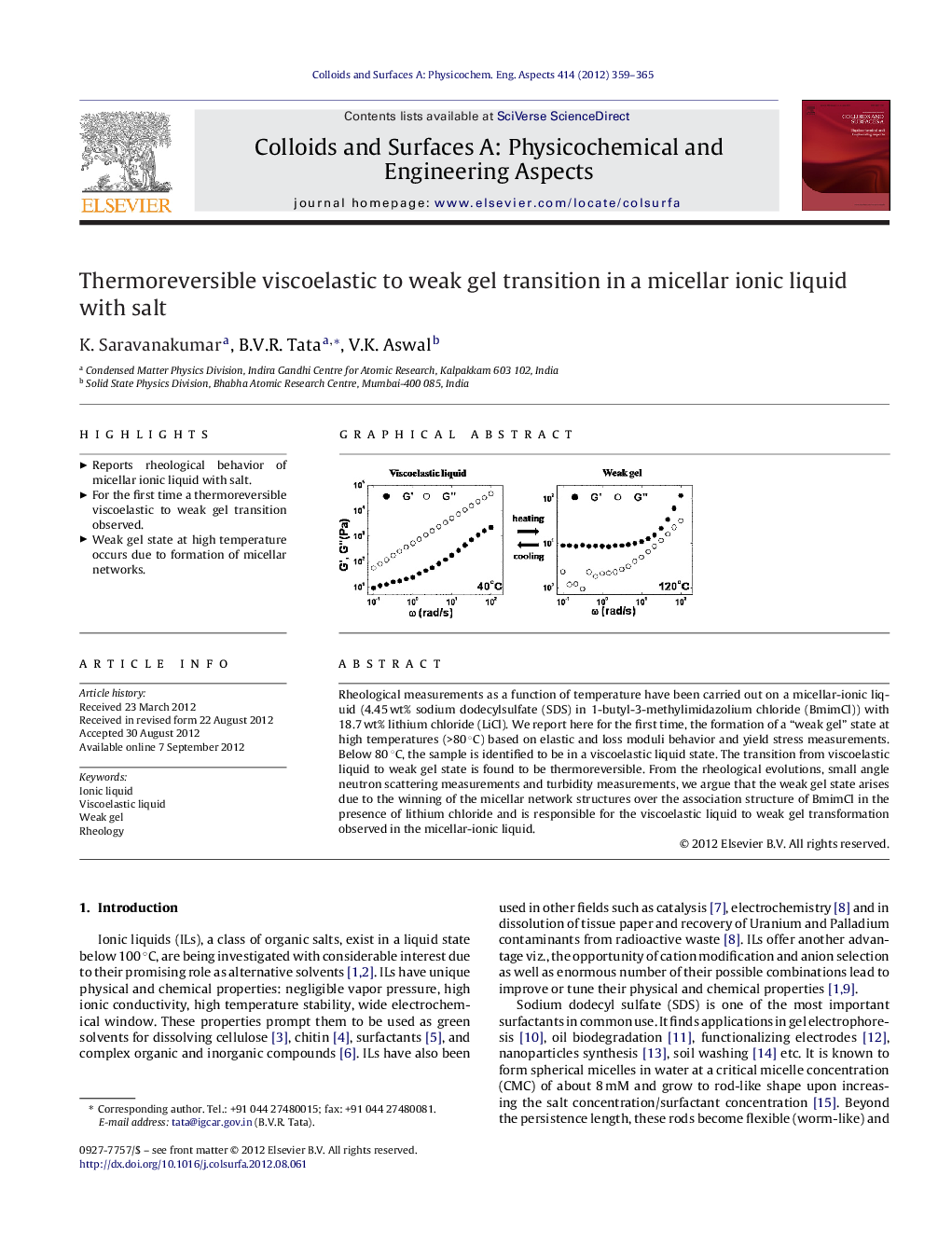
Rheological measurements as a function of temperature have been carried out on a micellar-ionic liquid (4.45 wt% sodium dodecylsulfate (SDS) in 1-butyl-3-methylimidazolium chloride (BmimCl)) with 18.7 wt% lithium chloride (LiCl). We report here for the first time, the formation of a “weak gel” state at high temperatures (>80 °C) based on elastic and loss moduli behavior and yield stress measurements. Below 80 °C, the sample is identified to be in a viscoelastic liquid state. The transition from viscoelastic liquid to weak gel state is found to be thermoreversible. From the rheological evolutions, small angle neutron scattering measurements and turbidity measurements, we argue that the weak gel state arises due to the winning of the micellar network structures over the association structure of BmimCl in the presence of lithium chloride and is responsible for the viscoelastic liquid to weak gel transformation observed in the micellar-ionic liquid.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Reports rheological behavior of micellar ionic liquid with salt. ► For the first time a thermoreversible viscoelastic to weak gel transition observed. ► Weak gel state at high temperature occurs due to formation of micellar networks.