| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606304 | Journal of Colloid and Interface Science | 2016 | 8 Pages |
•Template immobilization strategy combined with surface imprinting technique to imprint protein.•Directly aldehyde-functionalized magnetic nanoparticles utilized as supports to simplify the preparation procedure.•Macromolecule gelatin used as functional monomer to enhance biocompatibility of imprinted polymers.•Specific removal of target protein from real biological sample.
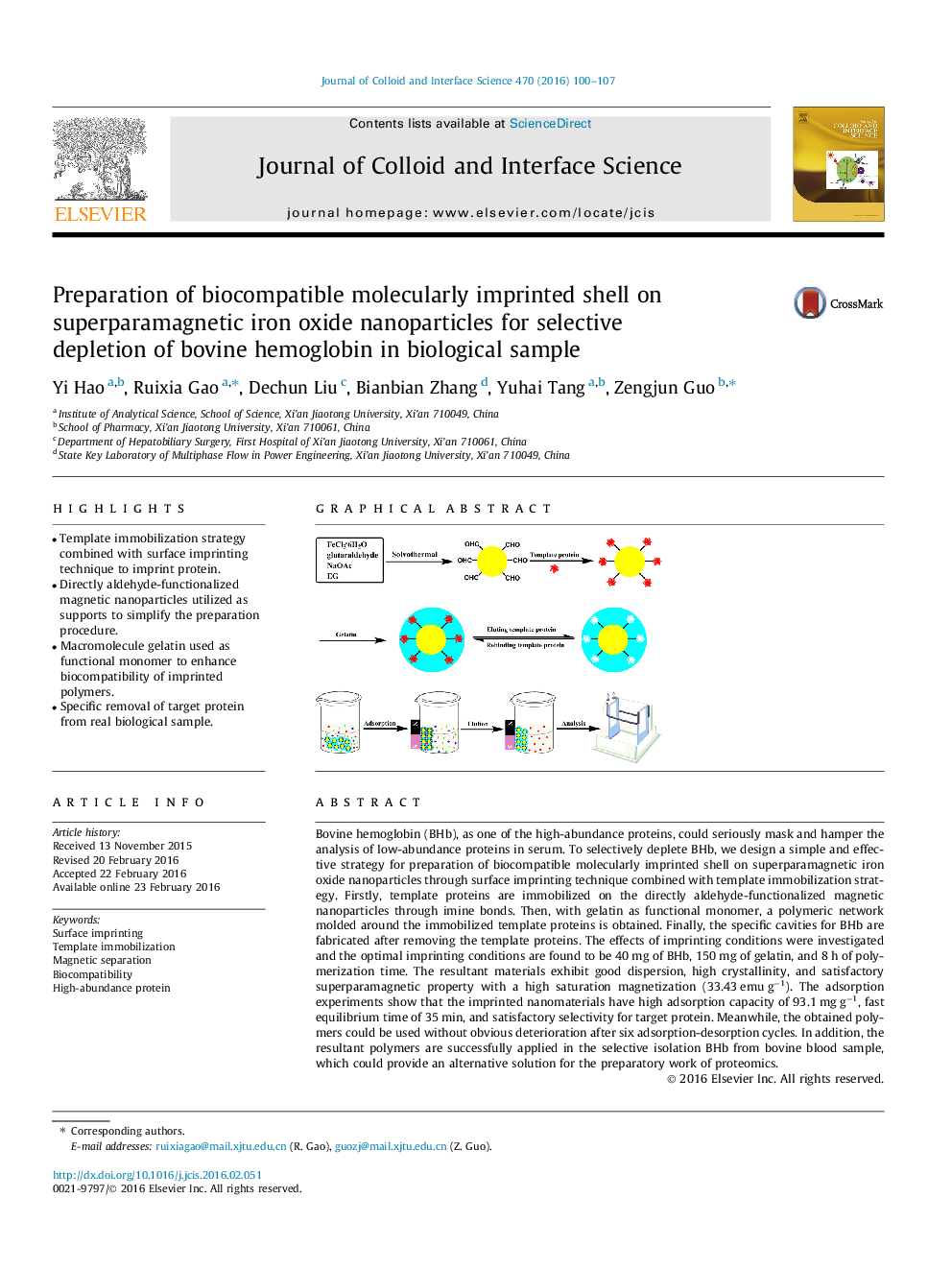
Bovine hemoglobin (BHb), as one of the high-abundance proteins, could seriously mask and hamper the analysis of low-abundance proteins in serum. To selectively deplete BHb, we design a simple and effective strategy for preparation of biocompatible molecularly imprinted shell on superparamagnetic iron oxide nanoparticles through surface imprinting technique combined with template immobilization strategy. Firstly, template proteins are immobilized on the directly aldehyde-functionalized magnetic nanoparticles through imine bonds. Then, with gelatin as functional monomer, a polymeric network molded around the immobilized template proteins is obtained. Finally, the specific cavities for BHb are fabricated after removing the template proteins. The effects of imprinting conditions were investigated and the optimal imprinting conditions are found to be 40 mg of BHb, 150 mg of gelatin, and 8 h of polymerization time. The resultant materials exhibit good dispersion, high crystallinity, and satisfactory superparamagnetic property with a high saturation magnetization (33.43 emu g−1). The adsorption experiments show that the imprinted nanomaterials have high adsorption capacity of 93.1 mg g−1, fast equilibrium time of 35 min, and satisfactory selectivity for target protein. Meanwhile, the obtained polymers could be used without obvious deterioration after six adsorption-desorption cycles. In addition, the resultant polymers are successfully applied in the selective isolation BHb from bovine blood sample, which could provide an alternative solution for the preparatory work of proteomics.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (147 K)Download as PowerPoint slide