| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606379 | Journal of Colloid and Interface Science | 2016 | 9 Pages |
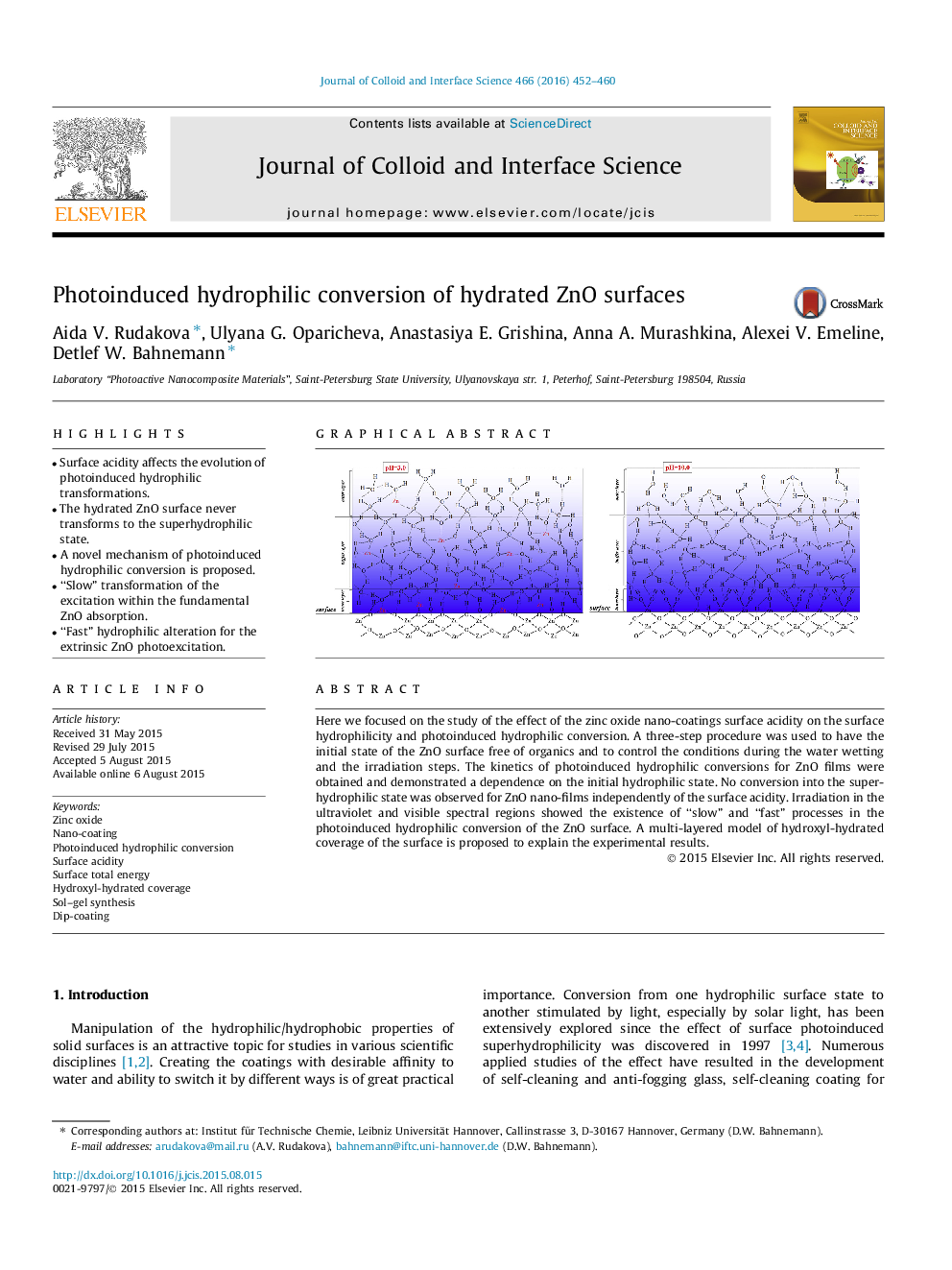
•Surface acidity affects the evolution of photoinduced hydrophilic transformations.•The hydrated ZnO surface never transforms to the superhydrophilic state.•A novel mechanism of photoinduced hydrophilic conversion is proposed.•“Slow” transformation of the excitation within the fundamental ZnO absorption.•“Fast” hydrophilic alteration for the extrinsic ZnO photoexcitation.
Here we focused on the study of the effect of the zinc oxide nano-coatings surface acidity on the surface hydrophilicity and photoinduced hydrophilic conversion. A three-step procedure was used to have the initial state of the ZnO surface free of organics and to control the conditions during the water wetting and the irradiation steps. The kinetics of photoinduced hydrophilic conversions for ZnO films were obtained and demonstrated a dependence on the initial hydrophilic state. No conversion into the superhydrophilic state was observed for ZnO nano-films independently of the surface acidity. Irradiation in the ultraviolet and visible spectral regions showed the existence of “slow” and “fast” processes in the photoinduced hydrophilic conversion of the ZnO surface. A multi-layered model of hydroxyl-hydrated coverage of the surface is proposed to explain the experimental results.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (268 K)Download as PowerPoint slide