| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606390 | Journal of Colloid and Interface Science | 2016 | 7 Pages |
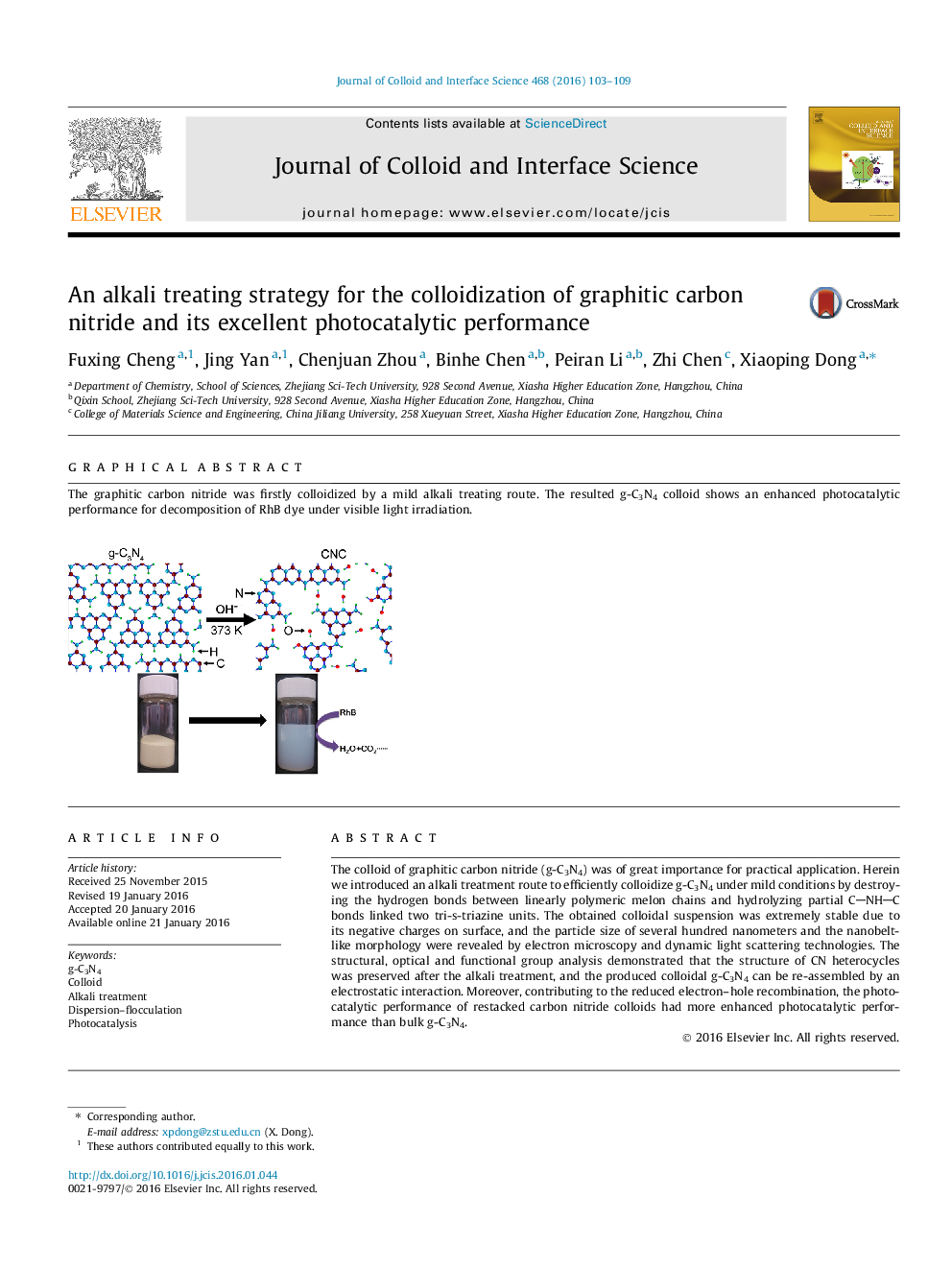
The colloid of graphitic carbon nitride (g-C3N4) was of great importance for practical application. Herein we introduced an alkali treatment route to efficiently colloidize g-C3N4 under mild conditions by destroying the hydrogen bonds between linearly polymeric melon chains and hydrolyzing partial CNHC bonds linked two tri-s-triazine units. The obtained colloidal suspension was extremely stable due to its negative charges on surface, and the particle size of several hundred nanometers and the nanobelt-like morphology were revealed by electron microscopy and dynamic light scattering technologies. The structural, optical and functional group analysis demonstrated that the structure of CN heterocycles was preserved after the alkali treatment, and the produced colloidal g-C3N4 can be re-assembled by an electrostatic interaction. Moreover, contributing to the reduced electron–hole recombination, the photocatalytic performance of restacked carbon nitride colloids had more enhanced photocatalytic performance than bulk g-C3N4.
Graphical abstractThe graphitic carbon nitride was firstly colloidized by a mild alkali treating route. The resulted g-C3N4 colloid shows an enhanced photocatalytic performance for decomposition of RhB dye under visible light irradiation.Figure optionsDownload full-size imageDownload high-quality image (153 K)Download as PowerPoint slide