| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606469 | Journal of Colloid and Interface Science | 2016 | 8 Pages |
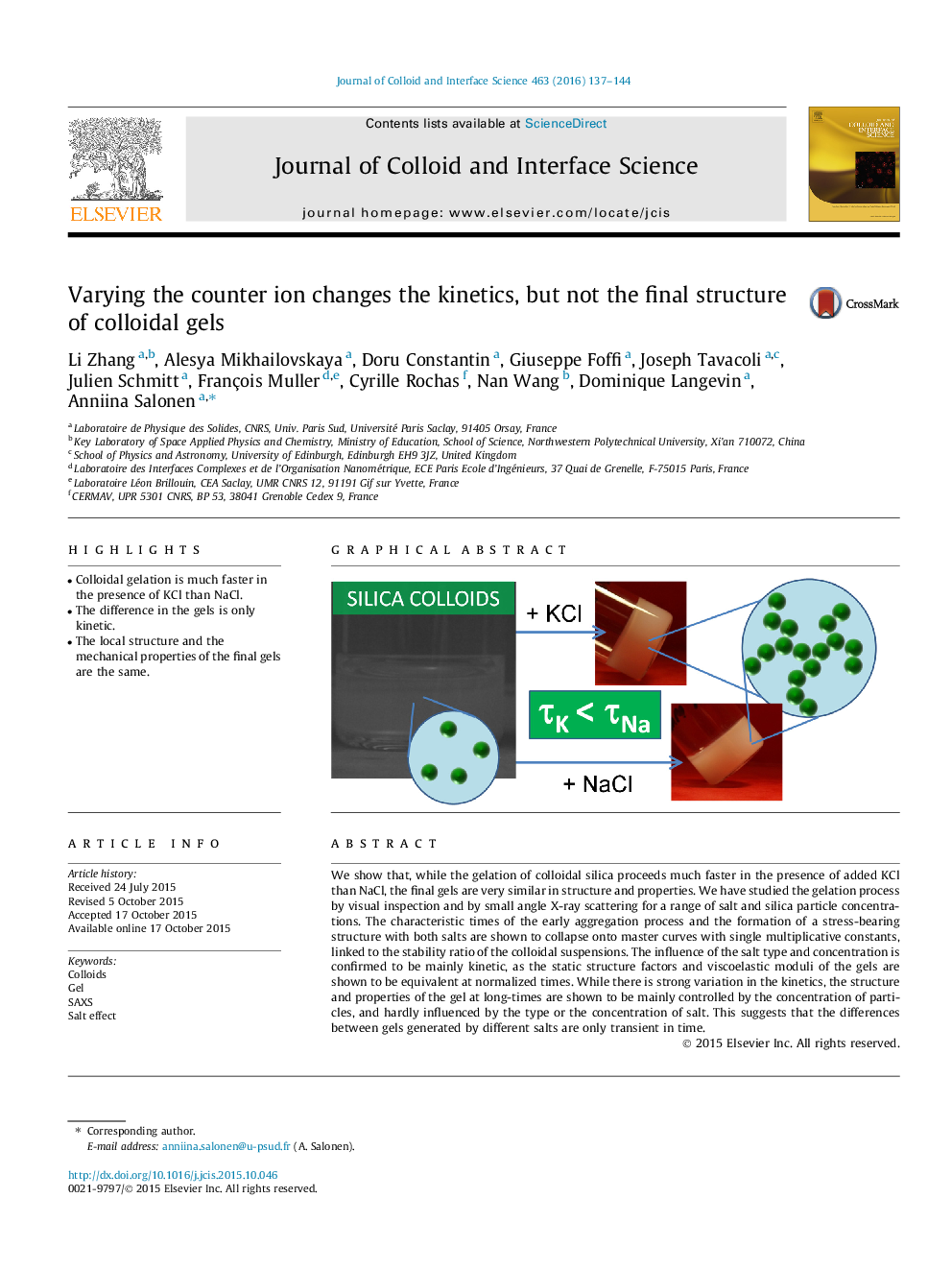
•Colloidal gelation is much faster in the presence of KCl than NaCl.•The difference in the gels is only kinetic.•The local structure and the mechanical properties of the final gels are the same.
We show that, while the gelation of colloidal silica proceeds much faster in the presence of added KCl than NaCl, the final gels are very similar in structure and properties. We have studied the gelation process by visual inspection and by small angle X-ray scattering for a range of salt and silica particle concentrations. The characteristic times of the early aggregation process and the formation of a stress-bearing structure with both salts are shown to collapse onto master curves with single multiplicative constants, linked to the stability ratio of the colloidal suspensions. The influence of the salt type and concentration is confirmed to be mainly kinetic, as the static structure factors and viscoelastic moduli of the gels are shown to be equivalent at normalized times. While there is strong variation in the kinetics, the structure and properties of the gel at long-times are shown to be mainly controlled by the concentration of particles, and hardly influenced by the type or the concentration of salt. This suggests that the differences between gels generated by different salts are only transient in time.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (81 K)Download as PowerPoint slide