| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606480 | Journal of Colloid and Interface Science | 2016 | 9 Pages |
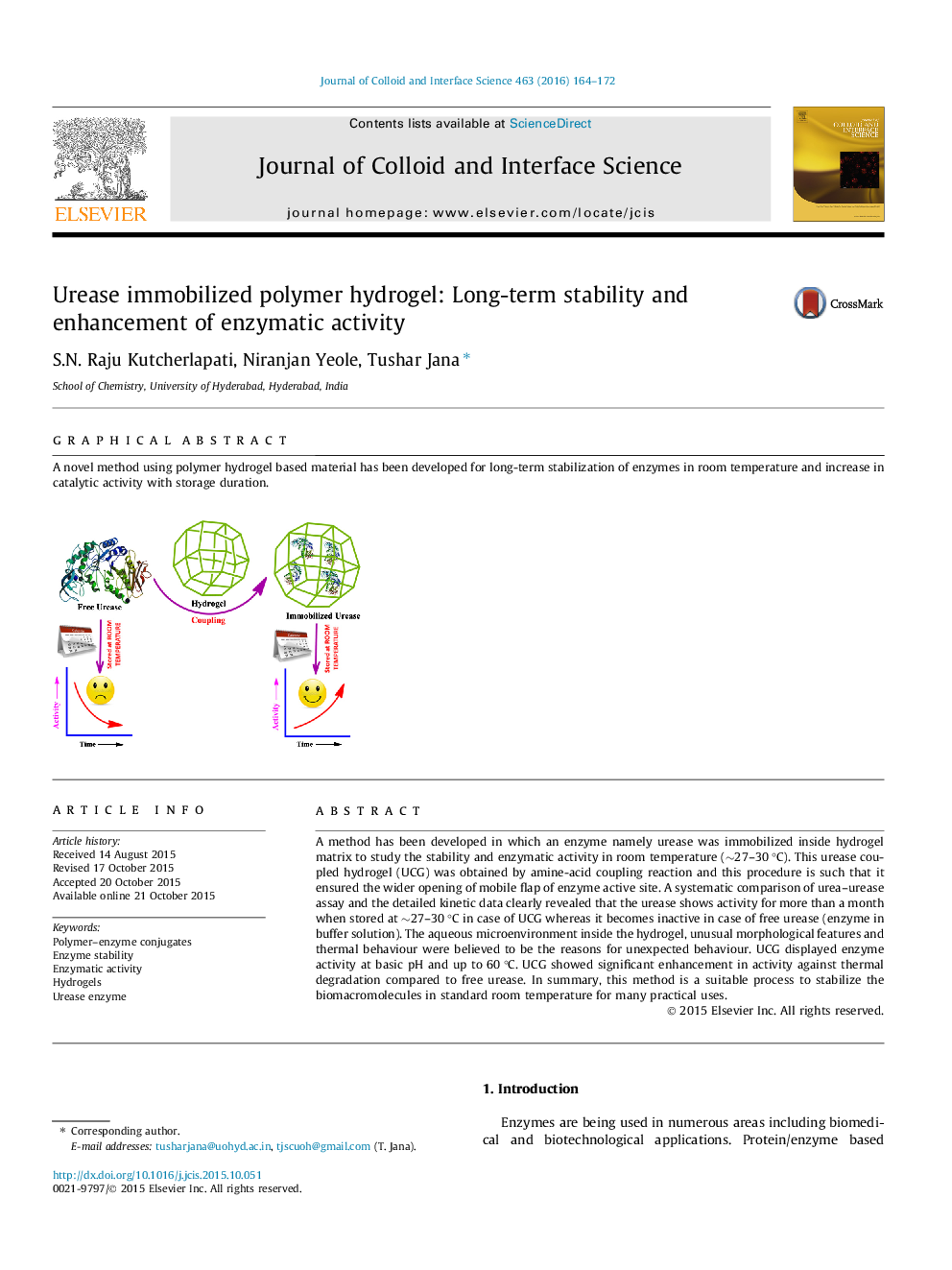
A method has been developed in which an enzyme namely urease was immobilized inside hydrogel matrix to study the stability and enzymatic activity in room temperature (∼27–30 °C). This urease coupled hydrogel (UCG) was obtained by amine-acid coupling reaction and this procedure is such that it ensured the wider opening of mobile flap of enzyme active site. A systematic comparison of urea–urease assay and the detailed kinetic data clearly revealed that the urease shows activity for more than a month when stored at ∼27–30 °C in case of UCG whereas it becomes inactive in case of free urease (enzyme in buffer solution). The aqueous microenvironment inside the hydrogel, unusual morphological features and thermal behaviour were believed to be the reasons for unexpected behaviour. UCG displayed enzyme activity at basic pH and up to 60 °C. UCG showed significant enhancement in activity against thermal degradation compared to free urease. In summary, this method is a suitable process to stabilize the biomacromolecules in standard room temperature for many practical uses.
Graphical abstractA novel method using polymer hydrogel based material has been developed for long-term stabilization of enzymes in room temperature and increase in catalytic activity with storage duration.Figure optionsDownload full-size imageDownload high-quality image (157 K)Download as PowerPoint slide