| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 606971 | Journal of Colloid and Interface Science | 2015 | 9 Pages |
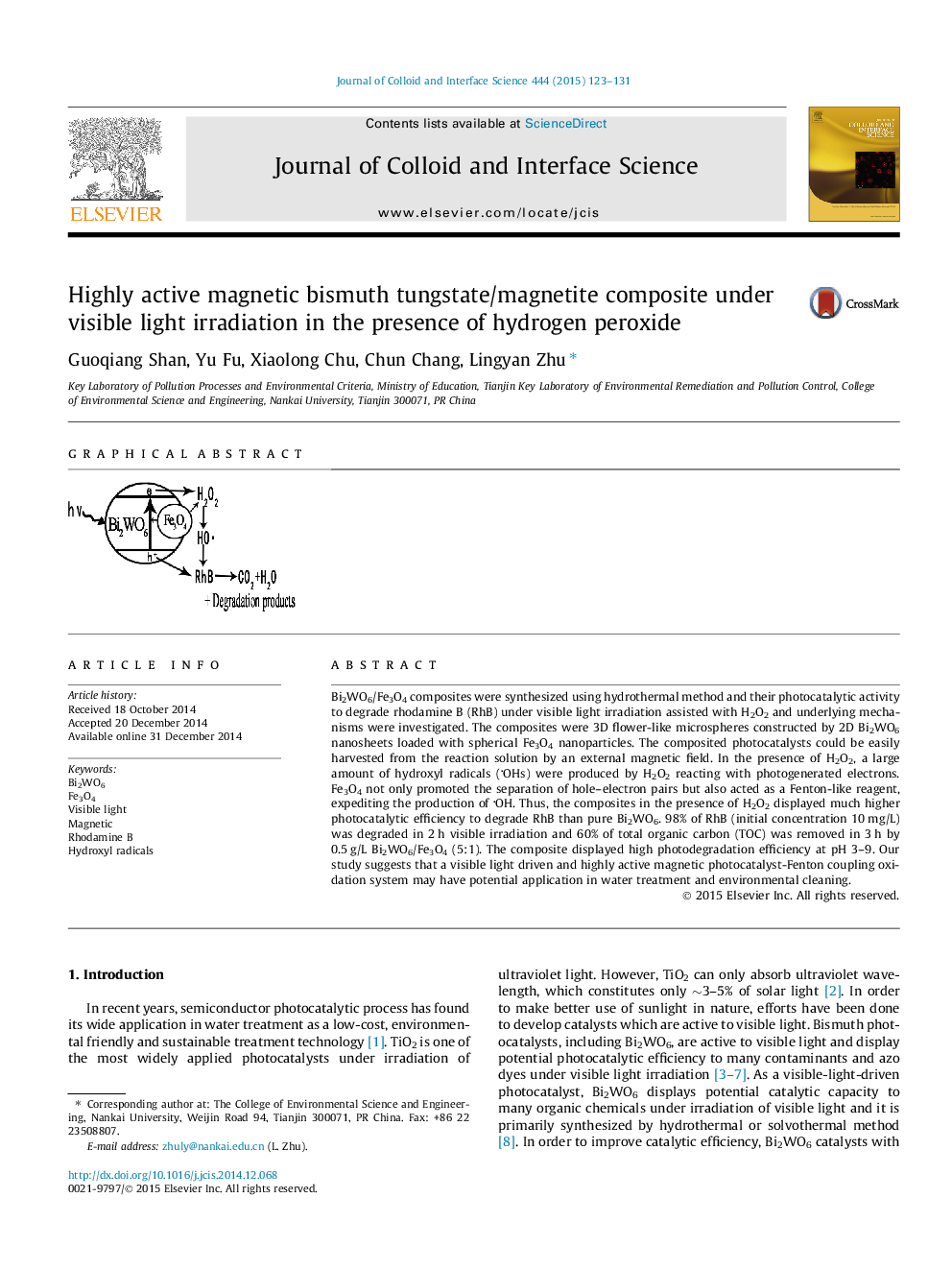
Bi2WO6/Fe3O4 composites were synthesized using hydrothermal method and their photocatalytic activity to degrade rhodamine B (RhB) under visible light irradiation assisted with H2O2 and underlying mechanisms were investigated. The composites were 3D flower-like microspheres constructed by 2D Bi2WO6 nanosheets loaded with spherical Fe3O4 nanoparticles. The composited photocatalysts could be easily harvested from the reaction solution by an external magnetic field. In the presence of H2O2, a large amount of hydroxyl radicals (OHs) were produced by H2O2 reacting with photogenerated electrons. Fe3O4 not only promoted the separation of hole–electron pairs but also acted as a Fenton-like reagent, expediting the production of OH. Thus, the composites in the presence of H2O2 displayed much higher photocatalytic efficiency to degrade RhB than pure Bi2WO6. 98% of RhB (initial concentration 10 mg/L) was degraded in 2 h visible irradiation and 60% of total organic carbon (TOC) was removed in 3 h by 0.5 g/L Bi2WO6/Fe3O4 (5:1). The composite displayed high photodegradation efficiency at pH 3–9. Our study suggests that a visible light driven and highly active magnetic photocatalyst-Fenton coupling oxidation system may have potential application in water treatment and environmental cleaning.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (54 K)Download as PowerPoint slide