| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10590473 | Bioorganic & Medicinal Chemistry Letters | 2015 | 6 Pages |
Abstract
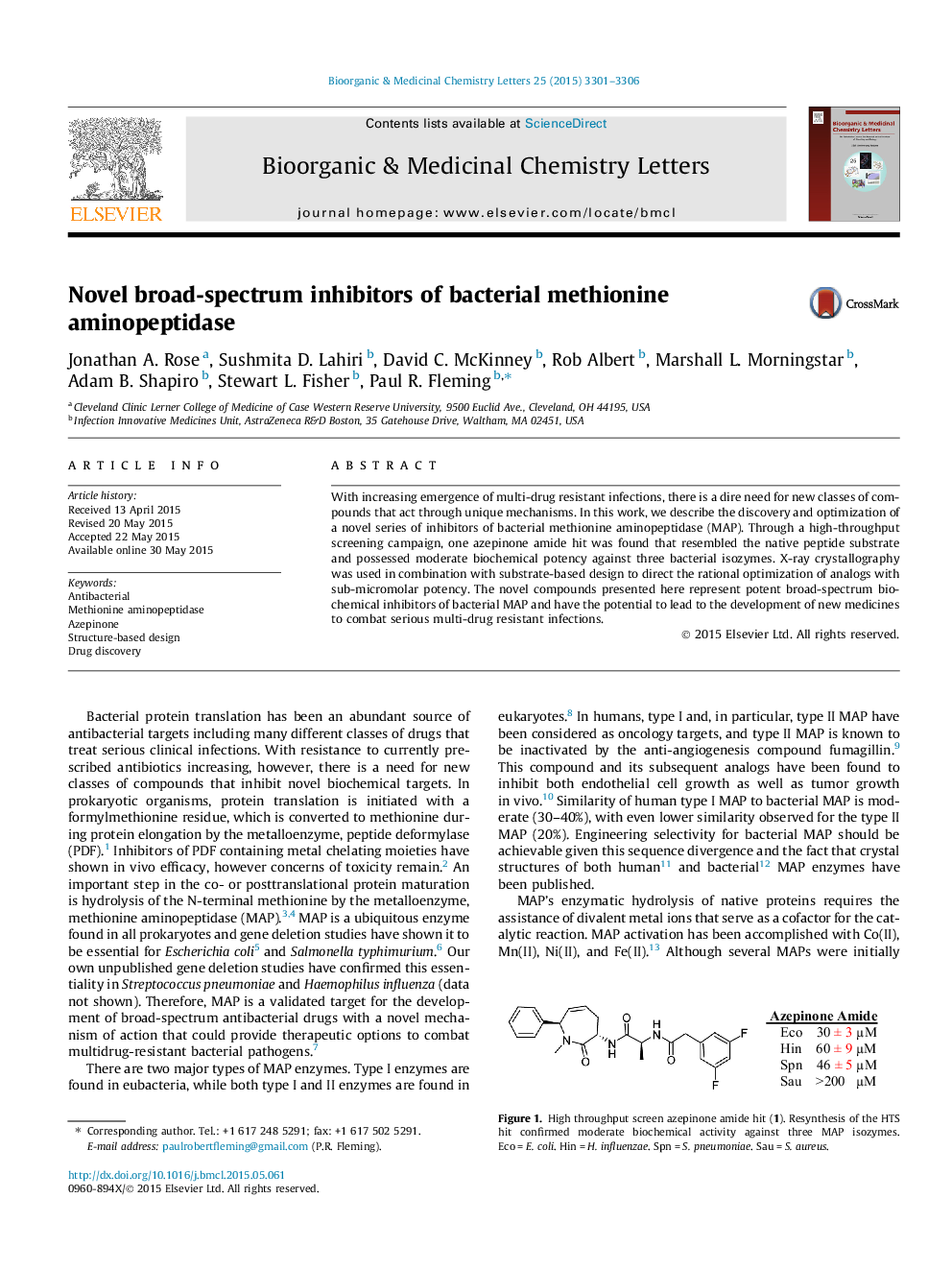
With increasing emergence of multi-drug resistant infections, there is a dire need for new classes of compounds that act through unique mechanisms. In this work, we describe the discovery and optimization of a novel series of inhibitors of bacterial methionine aminopeptidase (MAP). Through a high-throughput screening campaign, one azepinone amide hit was found that resembled the native peptide substrate and possessed moderate biochemical potency against three bacterial isozymes. X-ray crystallography was used in combination with substrate-based design to direct the rational optimization of analogs with sub-micromolar potency. The novel compounds presented here represent potent broad-spectrum biochemical inhibitors of bacterial MAP and have the potential to lead to the development of new medicines to combat serious multi-drug resistant infections.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Jonathan A. Rose, Sushmita D. Lahiri, David C. McKinney, Rob Albert, Marshall L. Morningstar, Adam B. Shapiro, Stewart L. Fisher, Paul R. Fleming,