| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10591847 | Bioorganic & Medicinal Chemistry Letters | 2014 | 4 Pages |
Abstract
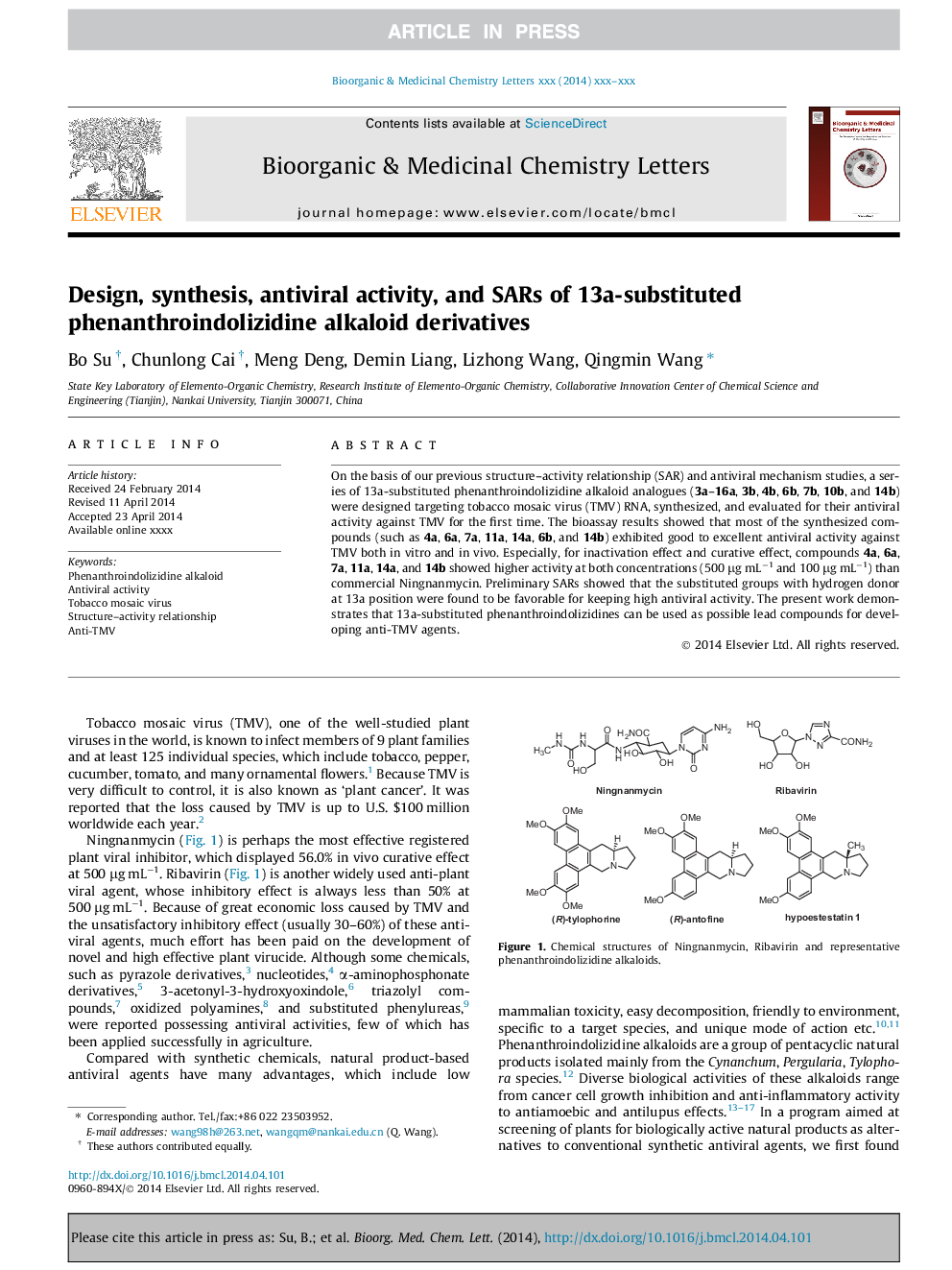
On the basis of our previous structure-activity relationship (SAR) and antiviral mechanism studies, a series of 13a-substituted phenanthroindolizidine alkaloid analogues (3a-16a, 3b, 4b, 6b, 7b, 10b, and 14b) were designed targeting tobacco mosaic virus (TMV) RNA, synthesized, and evaluated for their antiviral activity against TMV for the first time. The bioassay results showed that most of the synthesized compounds (such as 4a, 6a, 7a, 11a, 14a, 6b, and 14b) exhibited good to excellent antiviral activity against TMV both in vitro and in vivo. Especially, for inactivation effect and curative effect, compounds 4a, 6a, 7a, 11a, 14a, and 14b showed higher activity at both concentrations (500 μg mLâ1 and 100 μg mLâ1) than commercial Ningnanmycin. Preliminary SARs showed that the substituted groups with hydrogen donor at 13a position were found to be favorable for keeping high antiviral activity. The present work demonstrates that 13a-substituted phenanthroindolizidines can be used as possible lead compounds for developing anti-TMV agents.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Bo Su, Chunlong Cai, Meng Deng, Demin Liang, Lizhong Wang, Qingmin Wang,