| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10594843 | Bioorganic & Medicinal Chemistry Letters | 2014 | 5 Pages |
Abstract
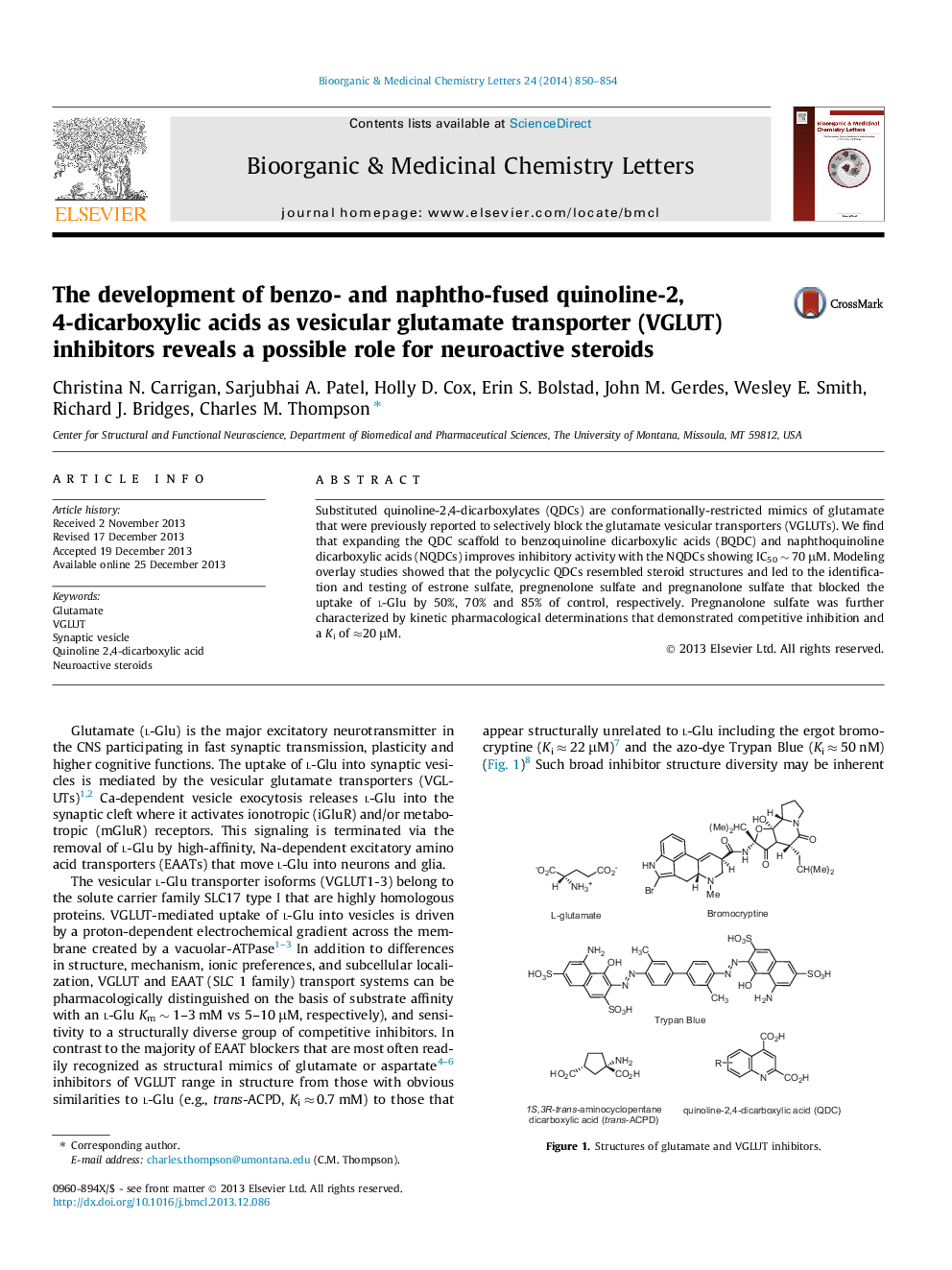
Substituted quinoline-2,4-dicarboxylates (QDCs) are conformationally-restricted mimics of glutamate that were previously reported to selectively block the glutamate vesicular transporters (VGLUTs). We find that expanding the QDC scaffold to benzoquinoline dicarboxylic acids (BQDC) and naphthoquinoline dicarboxylic acids (NQDCs) improves inhibitory activity with the NQDCs showing IC50 â¼Â 70 μM. Modeling overlay studies showed that the polycyclic QDCs resembled steroid structures and led to the identification and testing of estrone sulfate, pregnenolone sulfate and pregnanolone sulfate that blocked the uptake of l-Glu by 50%, 70% and 85% of control, respectively. Pregnanolone sulfate was further characterized by kinetic pharmacological determinations that demonstrated competitive inhibition and a Ki of â20 μM.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Christina N. Carrigan, Sarjubhai A. Patel, Holly D. Cox, Erin S. Bolstad, John M. Gerdes, Wesley E. Smith, Richard J. Bridges, Charles M. Thompson,