| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10595284 | Bioorganic & Medicinal Chemistry Letters | 2012 | 4 Pages |
Abstract
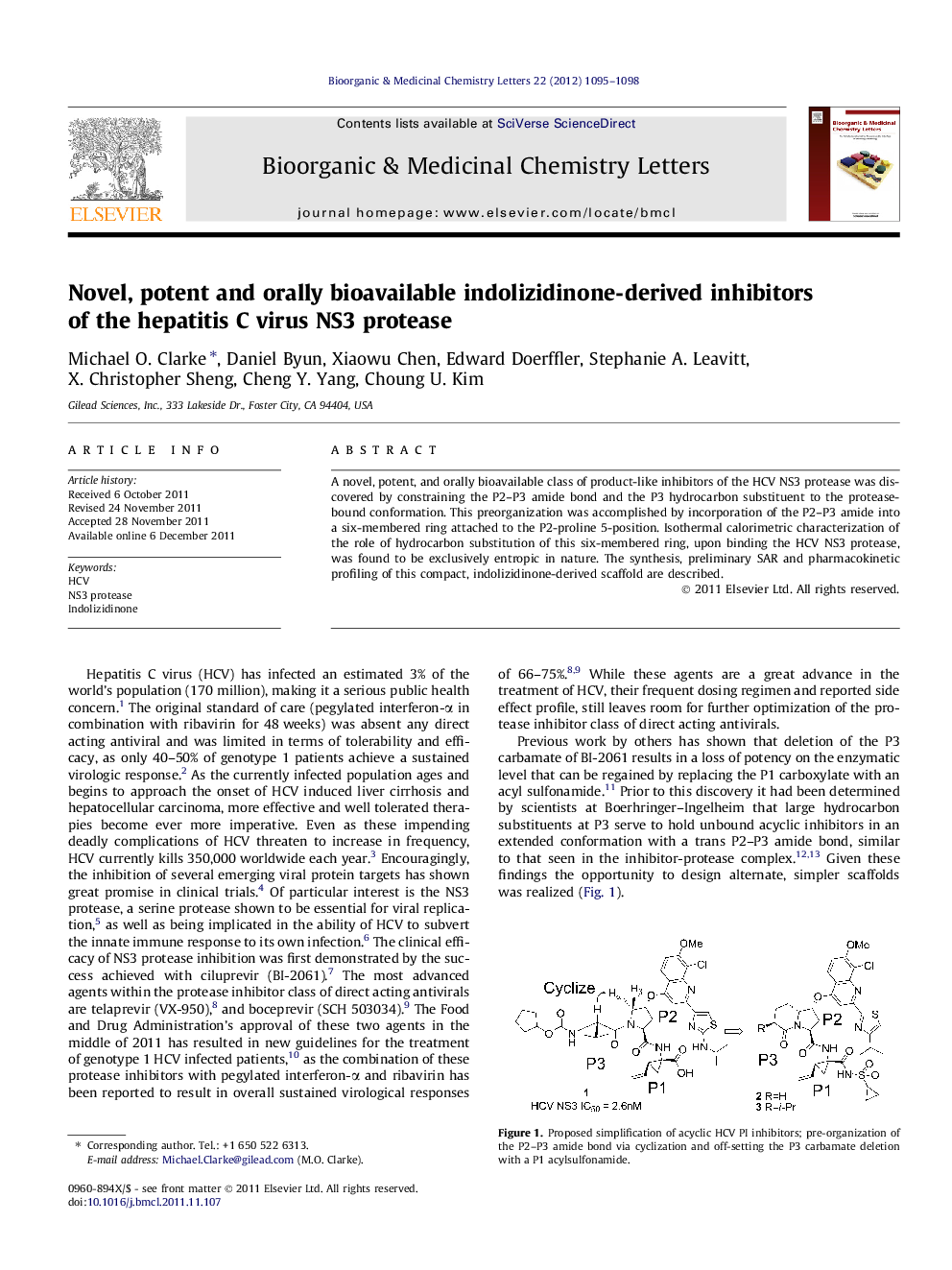
A novel, potent, and orally bioavailable class of product-like inhibitors of the HCV NS3 protease was discovered by constraining the P2-P3 amide bond and the P3 hydrocarbon substituent to the protease-bound conformation. This preorganization was accomplished by incorporation of the P2-P3 amide into a six-membered ring attached to the P2-proline 5-position. Isothermal calorimetric characterization of the role of hydrocarbon substitution of this six-membered ring, upon binding the HCV NS3 protease, was found to be exclusively entropic in nature. The synthesis, preliminary SAR and pharmacokinetic profiling of this compact, indolizidinone-derived scaffold are described.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Michael O. Clarke, Daniel Byun, Xiaowu Chen, Edward Doerffler, Stephanie A. Leavitt, X. Christopher Sheng, Cheng Y. Yang, Choung U. Kim,