| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10595913 | Bioorganic & Medicinal Chemistry Letters | 2013 | 5 Pages |
Abstract
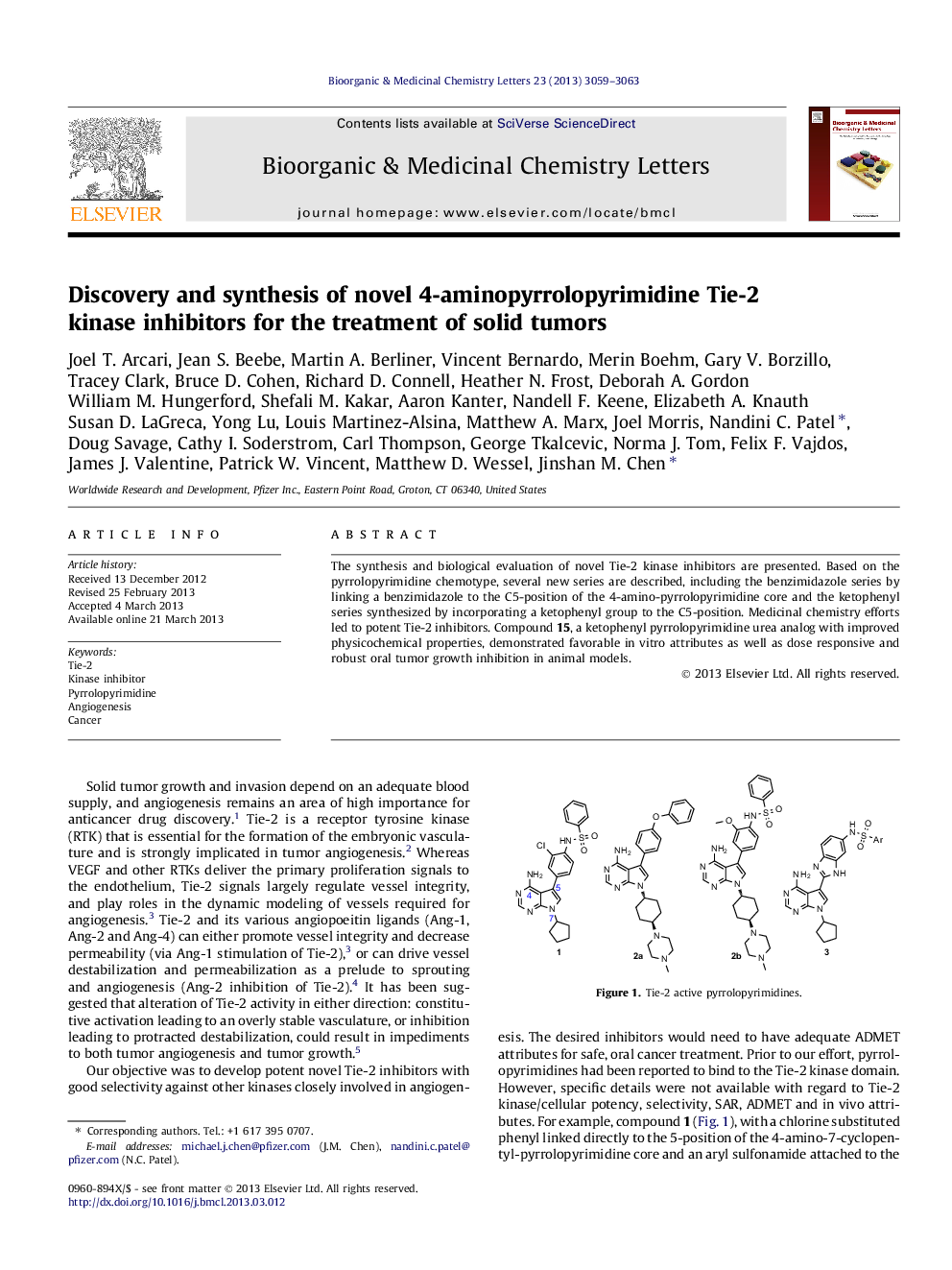
The synthesis and biological evaluation of novel Tie-2 kinase inhibitors are presented. Based on the pyrrolopyrimidine chemotype, several new series are described, including the benzimidazole series by linking a benzimidazole to the C5-position of the 4-amino-pyrrolopyrimidine core and the ketophenyl series synthesized by incorporating a ketophenyl group to the C5-position. Medicinal chemistry efforts led to potent Tie-2 inhibitors. Compound 15, a ketophenyl pyrrolopyrimidine urea analog with improved physicochemical properties, demonstrated favorable in vitro attributes as well as dose responsive and robust oral tumor growth inhibition in animal models.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Joel T. Arcari, Jean S. Beebe, Martin A. Berliner, Vincent Bernardo, Merin Boehm, Gary V. Borzillo, Tracey Clark, Bruce D. Cohen, Richard D. Connell, Heather N. Frost, Deborah A. Gordon, William M. Hungerford, Shefali M. Kakar, Aaron Kanter,