| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 10596530 | Bioorganic & Medicinal Chemistry Letters | 2012 | 5 Pages |
Abstract
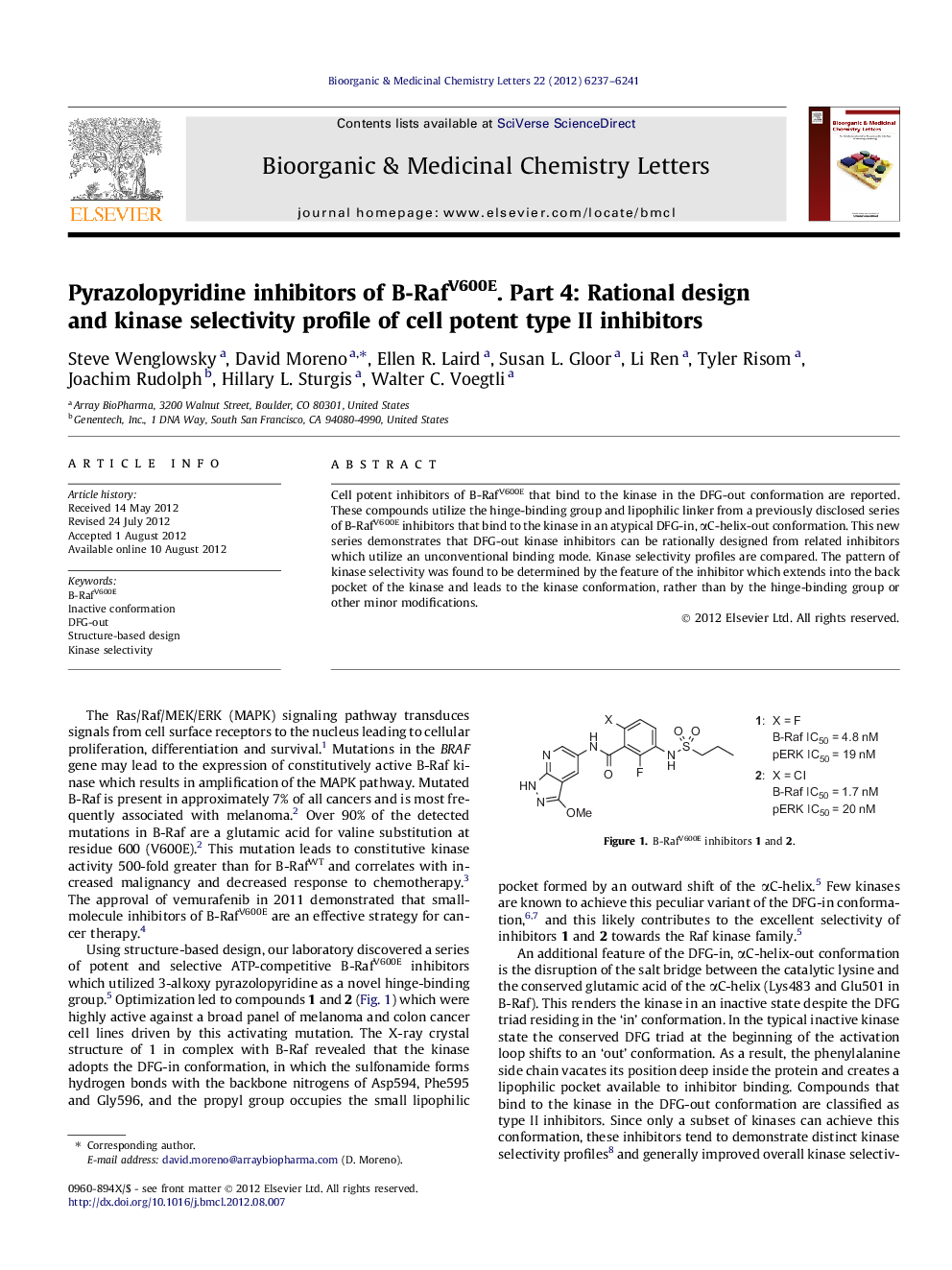
Cell potent inhibitors of B-RafV600E that bind to the kinase in the DFG-out conformation are reported. These compounds utilize the hinge-binding group and lipophilic linker from a previously disclosed series of B-RafV600E inhibitors that bind to the kinase in an atypical DFG-in, αC-helix-out conformation. This new series demonstrates that DFG-out kinase inhibitors can be rationally designed from related inhibitors which utilize an unconventional binding mode. Kinase selectivity profiles are compared. The pattern of kinase selectivity was found to be determined by the feature of the inhibitor which extends into the back pocket of the kinase and leads to the kinase conformation, rather than by the hinge-binding group or other minor modifications.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Steve Wenglowsky, David Moreno, Ellen R. Laird, Susan L. Gloor, Li Ren, Tyler Risom, Joachim Rudolph, Hillary L. Sturgis, Walter C. Voegtli,