| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1283566 | Journal of Power Sources | 2016 | 8 Pages |
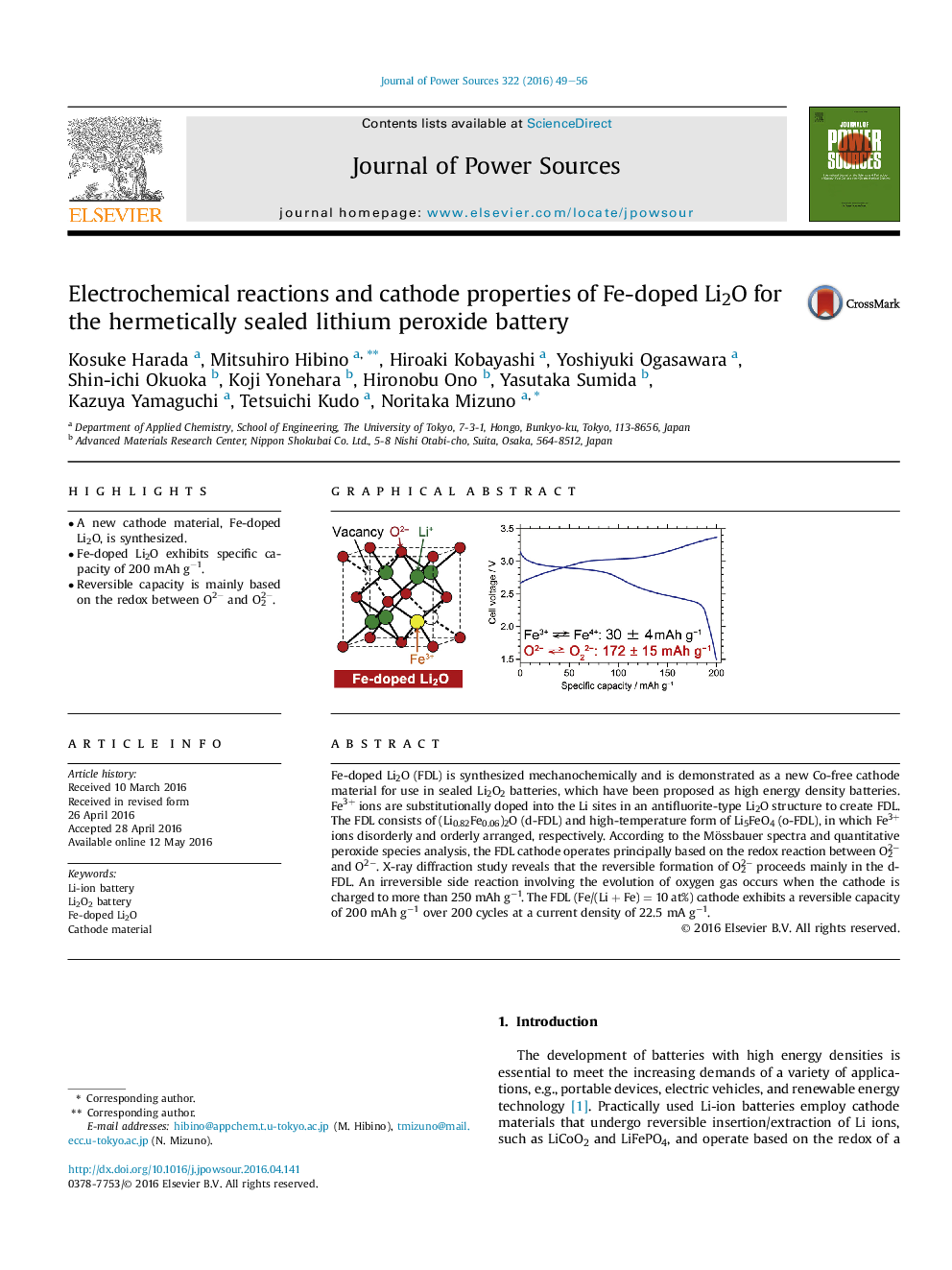
•A new cathode material, Fe-doped Li2O, is synthesized.•Fe-doped Li2O exhibits specific capacity of 200 mAh g−1.•Reversible capacity is mainly based on the redox between O2− and O22−.
Fe-doped Li2O (FDL) is synthesized mechanochemically and is demonstrated as a new Co-free cathode material for use in sealed Li2O2 batteries, which have been proposed as high energy density batteries. Fe3+ ions are substitutionally doped into the Li sites in an antifluorite-type Li2O structure to create FDL. The FDL consists of (Li0.82Fe0.06)2O (d-FDL) and high-temperature form of Li5FeO4 (o-FDL), in which Fe3+ ions disorderly and orderly arranged, respectively. According to the Mössbauer spectra and quantitative peroxide species analysis, the FDL cathode operates principally based on the redox reaction between O22− and O2−. X-ray diffraction study reveals that the reversible formation of O22− proceeds mainly in the d-FDL. An irreversible side reaction involving the evolution of oxygen gas occurs when the cathode is charged to more than 250 mAh g−1. The FDL (Fe/(Li + Fe) = 10 at%) cathode exhibits a reversible capacity of 200 mAh g−1 over 200 cycles at a current density of 22.5 mA g−1.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide