| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1283844 | Journal of Power Sources | 2015 | 10 Pages |
•DFT was used to study the role and fate of the PES additive in a Li-ion battery.•A “reactive electrode model” may explain decomposition to OCS at high voltage.•The calculated potential for PES reduction matches formation cycle coulometric data.•The reduced Li2PES species reacts with PES and the solvent to form SEI components.•XPS results match the predicted SEI components at the negative electrode.
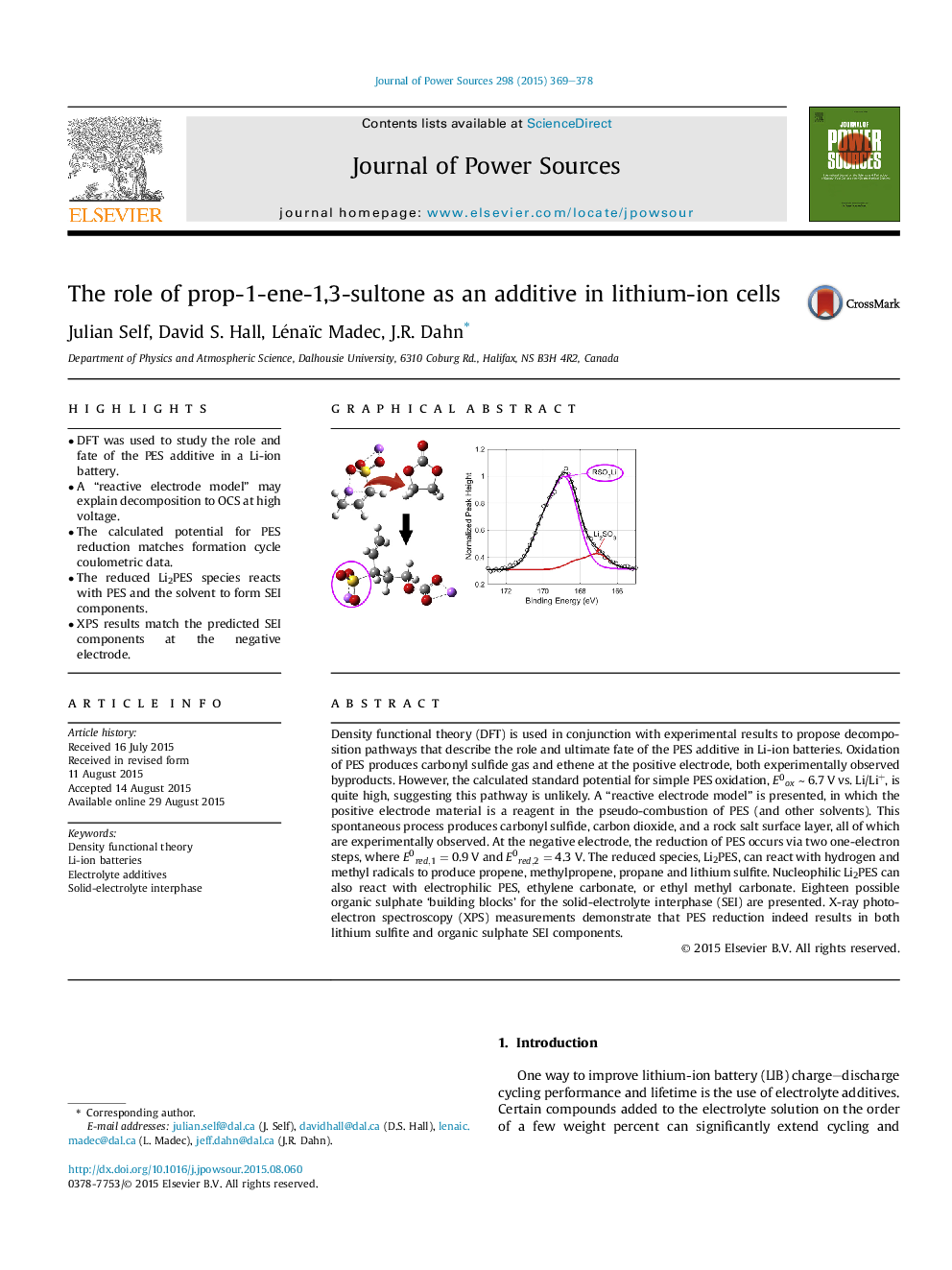
Density functional theory (DFT) is used in conjunction with experimental results to propose decomposition pathways that describe the role and ultimate fate of the PES additive in Li-ion batteries. Oxidation of PES produces carbonyl sulfide gas and ethene at the positive electrode, both experimentally observed byproducts. However, the calculated standard potential for simple PES oxidation, E0oxE0ox ∼ 6.7 V vs. Li/Li+, is quite high, suggesting this pathway is unlikely. A “reactive electrode model” is presented, in which the positive electrode material is a reagent in the pseudo-combustion of PES (and other solvents). This spontaneous process produces carbonyl sulfide, carbon dioxide, and a rock salt surface layer, all of which are experimentally observed. At the negative electrode, the reduction of PES occurs via two one-electron steps, where E0red,1E0red,1 = 0.9 V and E0red,2E0red,2 = 4.3 V. The reduced species, Li2PES, can react with hydrogen and methyl radicals to produce propene, methylpropene, propane and lithium sulfite. Nucleophilic Li2PES can also react with electrophilic PES, ethylene carbonate, or ethyl methyl carbonate. Eighteen possible organic sulphate ‘building blocks’ for the solid-electrolyte interphase (SEI) are presented. X-ray photoelectron spectroscopy (XPS) measurements demonstrate that PES reduction indeed results in both lithium sulfite and organic sulphate SEI components.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide