| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1284054 | Journal of Power Sources | 2014 | 7 Pages |
•We synthesize submicron LiNi0.5Mn1.5O4 by a facile route.•LiNi0.5Mn1.5O4 shows excellent rate performance and cycling stability.•LiNi0.5Mn1.5O4–MCMB cells show promising high-energy-density applications.
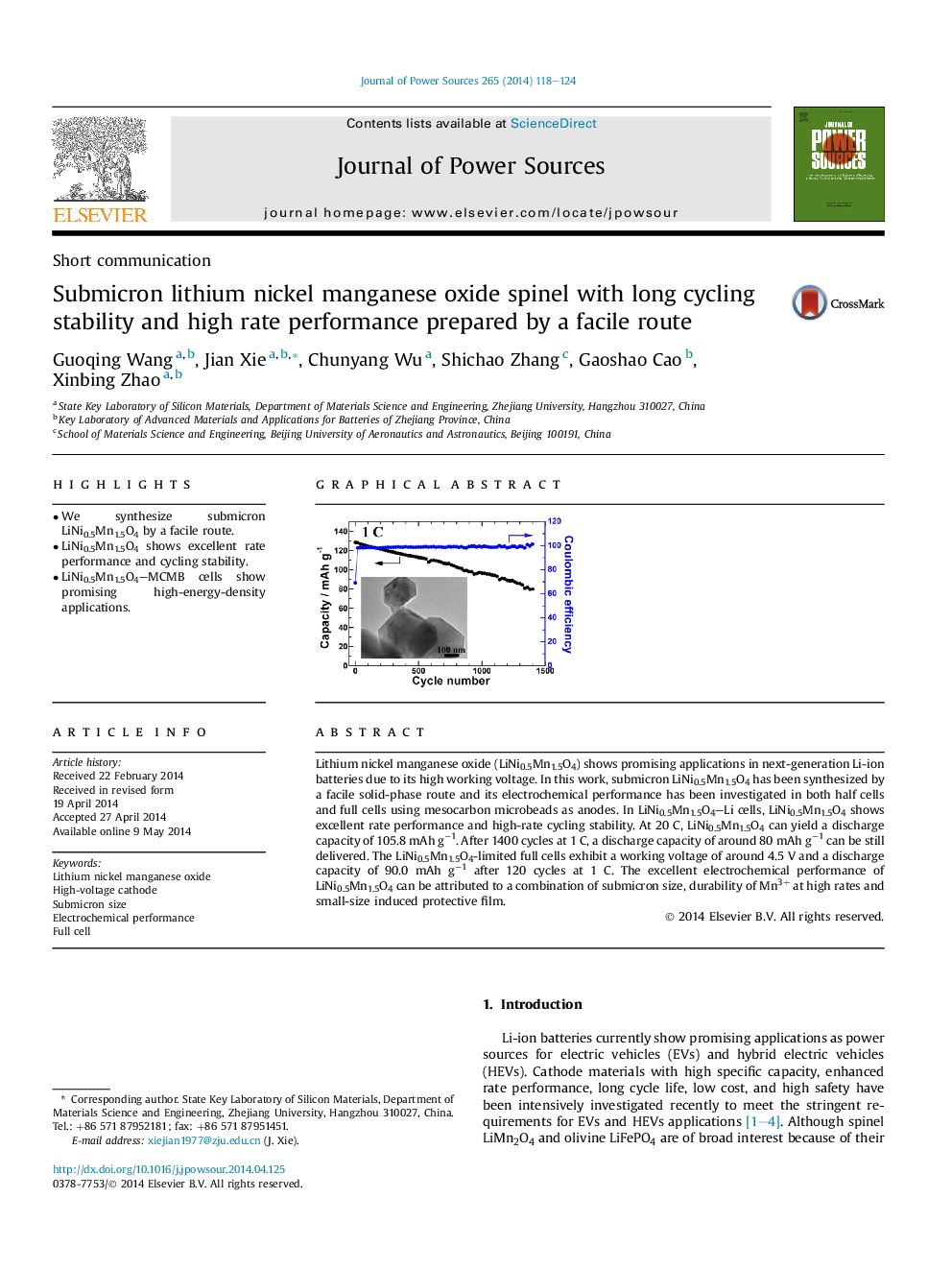
Lithium nickel manganese oxide (LiNi0.5Mn1.5O4) shows promising applications in next-generation Li-ion batteries due to its high working voltage. In this work, submicron LiNi0.5Mn1.5O4 has been synthesized by a facile solid-phase route and its electrochemical performance has been investigated in both half cells and full cells using mesocarbon microbeads as anodes. In LiNi0.5Mn1.5O4–Li cells, LiNi0.5Mn1.5O4 shows excellent rate performance and high-rate cycling stability. At 20 C, LiNi0.5Mn1.5O4 can yield a discharge capacity of 105.8 mAh g−1. After 1400 cycles at 1 C, a discharge capacity of around 80 mAh g−1 can be still delivered. The LiNi0.5Mn1.5O4-limited full cells exhibit a working voltage of around 4.5 V and a discharge capacity of 90.0 mAh g−1 after 120 cycles at 1 C. The excellent electrochemical performance of LiNi0.5Mn1.5O4 can be attributed to a combination of submicron size, durability of Mn3+ at high rates and small-size induced protective film.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide