| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1284217 | Journal of Power Sources | 2014 | 11 Pages |
•Design of the electrochemical cell for in-operando structural characterization of electrode materials for lithium-ion batteries.•Correlationship between electrochemical and structural changes in LixMn1.5Ni0.5O4.•Reversible cubic to tetragonal phase transition upon insertion/extraction of excess Li (x > 1) in LixMn1.5Ni0.5O4.
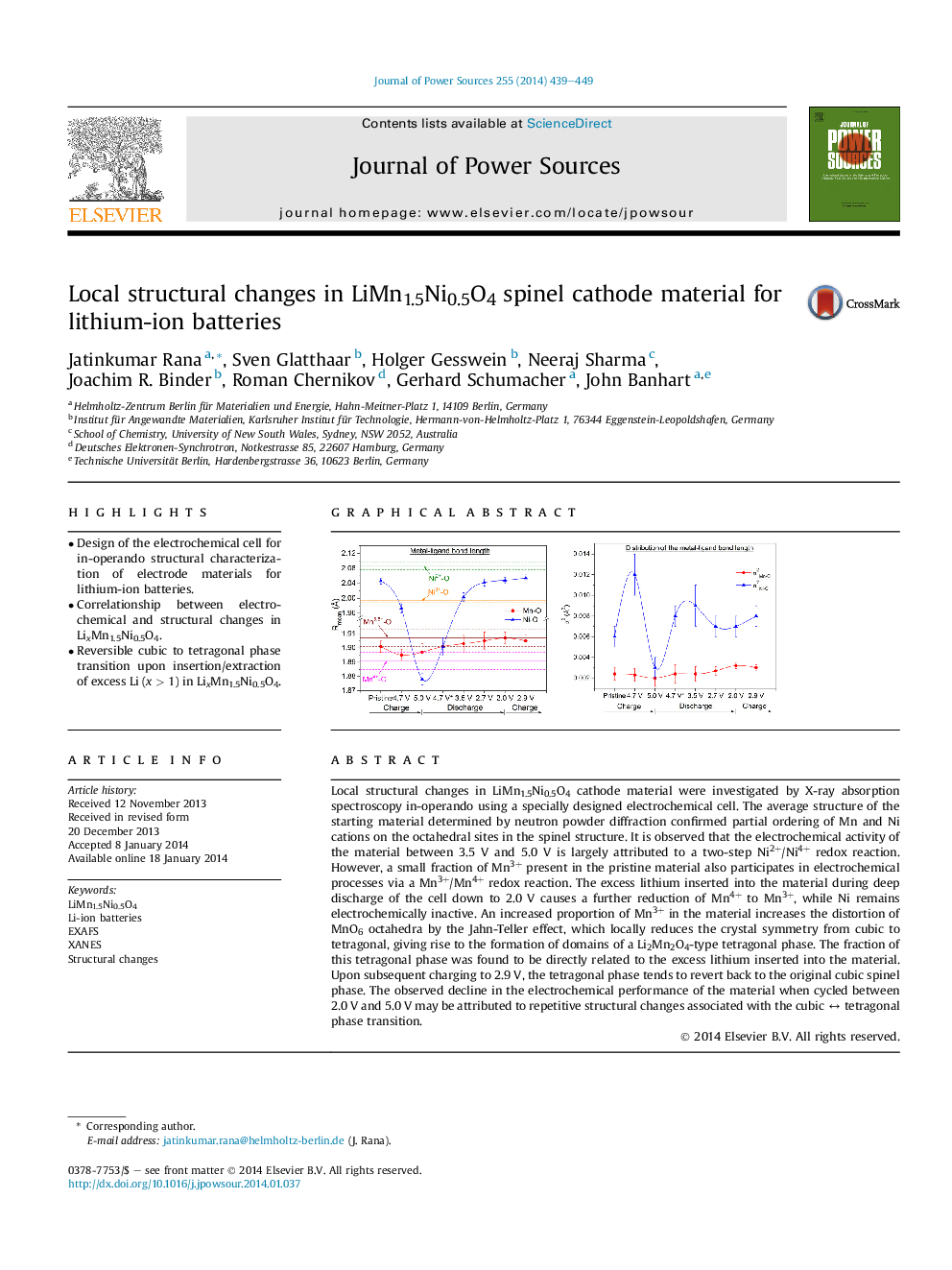
Local structural changes in LiMn1.5Ni0.5O4 cathode material were investigated by X-ray absorption spectroscopy in-operando using a specially designed electrochemical cell. The average structure of the starting material determined by neutron powder diffraction confirmed partial ordering of Mn and Ni cations on the octahedral sites in the spinel structure. It is observed that the electrochemical activity of the material between 3.5 V and 5.0 V is largely attributed to a two-step Ni2+/Ni4+ redox reaction. However, a small fraction of Mn3+ present in the pristine material also participates in electrochemical processes via a Mn3+/Mn4+ redox reaction. The excess lithium inserted into the material during deep discharge of the cell down to 2.0 V causes a further reduction of Mn4+ to Mn3+, while Ni remains electrochemically inactive. An increased proportion of Mn3+ in the material increases the distortion of MnO6 octahedra by the Jahn-Teller effect, which locally reduces the crystal symmetry from cubic to tetragonal, giving rise to the formation of domains of a Li2Mn2O4-type tetragonal phase. The fraction of this tetragonal phase was found to be directly related to the excess lithium inserted into the material. Upon subsequent charging to 2.9 V, the tetragonal phase tends to revert back to the original cubic spinel phase. The observed decline in the electrochemical performance of the material when cycled between 2.0 V and 5.0 V may be attributed to repetitive structural changes associated with the cubic ↔ tetragonal phase transition.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide