| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1284395 | Journal of Power Sources | 2013 | 6 Pages |
•VRFB cell has been constructed and the electrochemical performances have been studied.•V3+ to V4+ electron transfer state has been obtained.•Dimensionally stable electro materials Ti/IrO2:Ta2O5 are prepared and studied.•Energy density 33 Wh L−1 has been achieved for DSA electrodes.•The columbic, voltage and energy efficiencies of Ti/IrO2:Ta2O5 have been estimated and compared with graphite electrode.
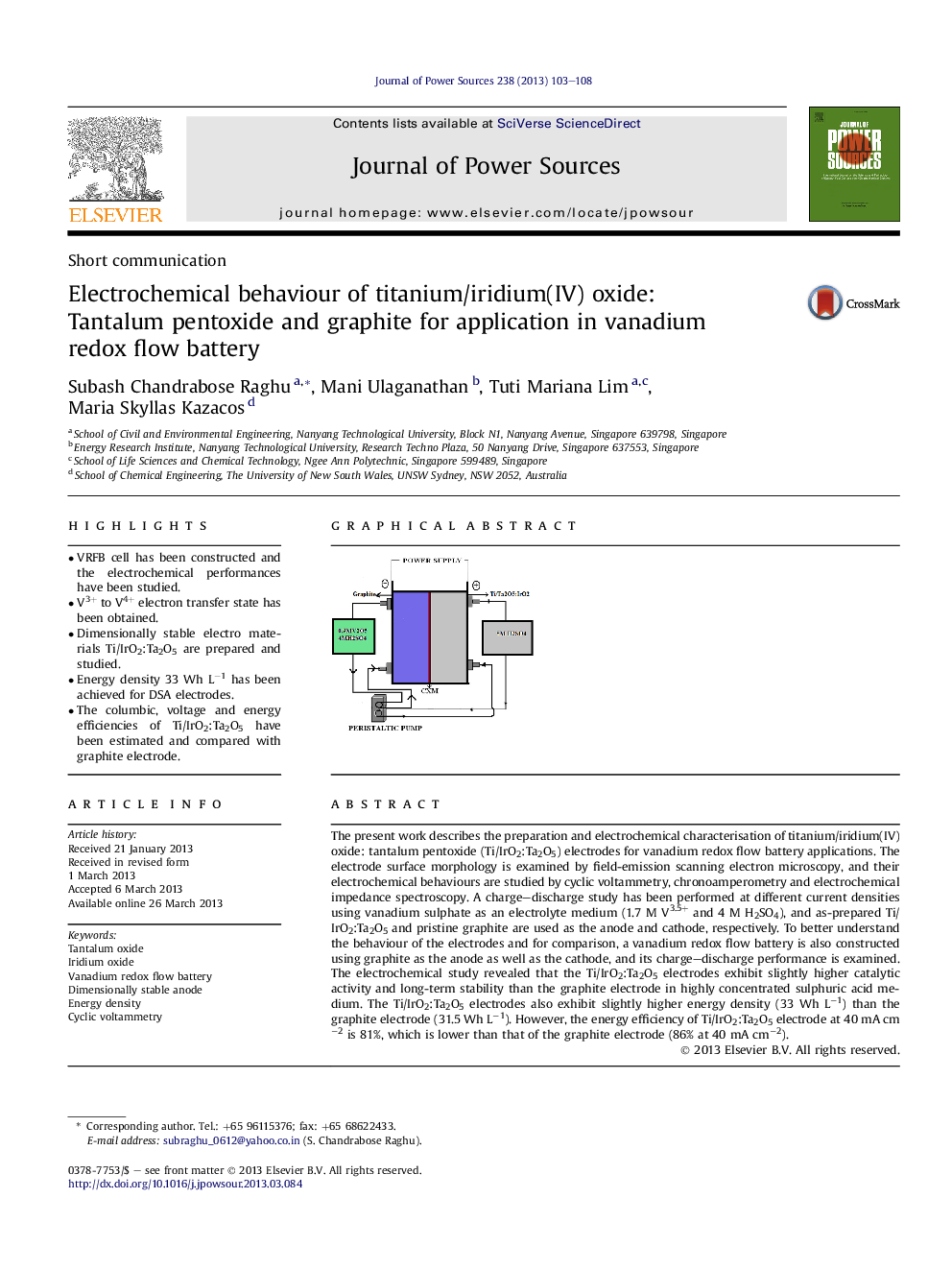
The present work describes the preparation and electrochemical characterisation of titanium/iridium(IV) oxide: tantalum pentoxide (Ti/IrO2:Ta2O5) electrodes for vanadium redox flow battery applications. The electrode surface morphology is examined by field-emission scanning electron microscopy, and their electrochemical behaviours are studied by cyclic voltammetry, chronoamperometry and electrochemical impedance spectroscopy. A charge–discharge study has been performed at different current densities using vanadium sulphate as an electrolyte medium (1.7 M V3.5+ and 4 M H2SO4), and as-prepared Ti/IrO2:Ta2O5 and pristine graphite are used as the anode and cathode, respectively. To better understand the behaviour of the electrodes and for comparison, a vanadium redox flow battery is also constructed using graphite as the anode as well as the cathode, and its charge–discharge performance is examined. The electrochemical study revealed that the Ti/IrO2:Ta2O5 electrodes exhibit slightly higher catalytic activity and long-term stability than the graphite electrode in highly concentrated sulphuric acid medium. The Ti/IrO2:Ta2O5 electrodes also exhibit slightly higher energy density (33 Wh L−1) than the graphite electrode (31.5 Wh L−1). However, the energy efficiency of Ti/IrO2:Ta2O5 electrode at 40 mA cm−2 is 81%, which is lower than that of the graphite electrode (86% at 40 mA cm−2).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide