| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1284465 | Journal of Power Sources | 2012 | 5 Pages |
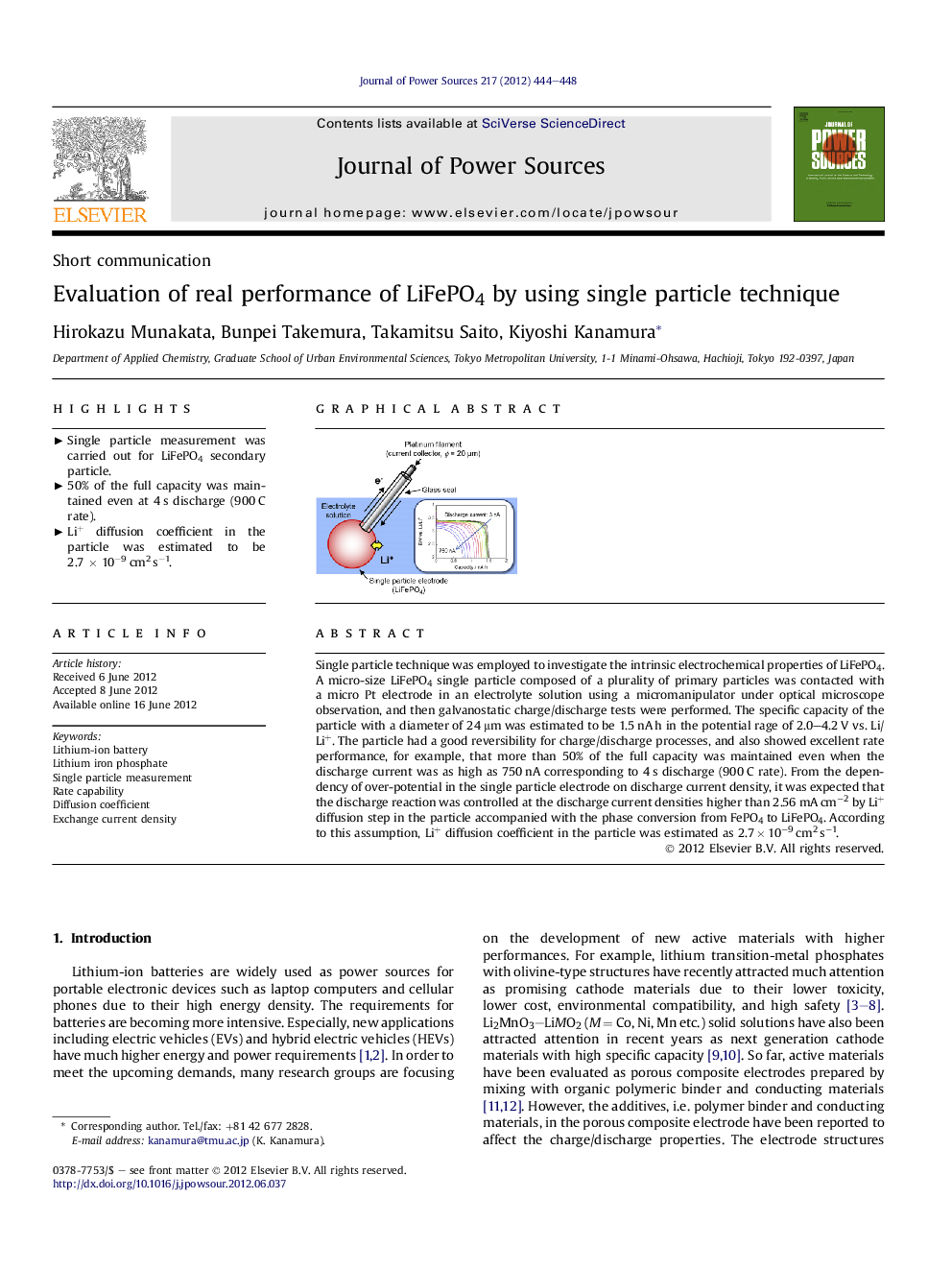
Single particle technique was employed to investigate the intrinsic electrochemical properties of LiFePO4. A micro-size LiFePO4 single particle composed of a plurality of primary particles was contacted with a micro Pt electrode in an electrolyte solution using a micromanipulator under optical microscope observation, and then galvanostatic charge/discharge tests were performed. The specific capacity of the particle with a diameter of 24 μm was estimated to be 1.5 nA h in the potential rage of 2.0–4.2 V vs. Li/Li+. The particle had a good reversibility for charge/discharge processes, and also showed excellent rate performance, for example, that more than 50% of the full capacity was maintained even when the discharge current was as high as 750 nA corresponding to 4 s discharge (900 C rate). From the dependency of over-potential in the single particle electrode on discharge current density, it was expected that the discharge reaction was controlled at the discharge current densities higher than 2.56 mA cm−2 by Li+ diffusion step in the particle accompanied with the phase conversion from FePO4 to LiFePO4. According to this assumption, Li+ diffusion coefficient in the particle was estimated as 2.7 × 10−9 cm2 s−1.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Single particle measurement was carried out for LiFePO4 secondary particle. ► 50% of the full capacity was maintained even at 4 s discharge (900 C rate). ► Li+ diffusion coefficient in the particle was estimated to be 2.7 × 10−9 cm2 s−1.