| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1285290 | Journal of Power Sources | 2016 | 7 Pages |
•AC/AC capacitors using Li2SO4 in water/methanol mixture operate down to −40 °C.•Hydrogen sorption in the negative electrode is thermodynamically quenched at −40 °C.•The system operates as a typical EDL capacitor at −40 °C.•The capacitor does not age during prolonged floating at 1.6 V and −40°C.
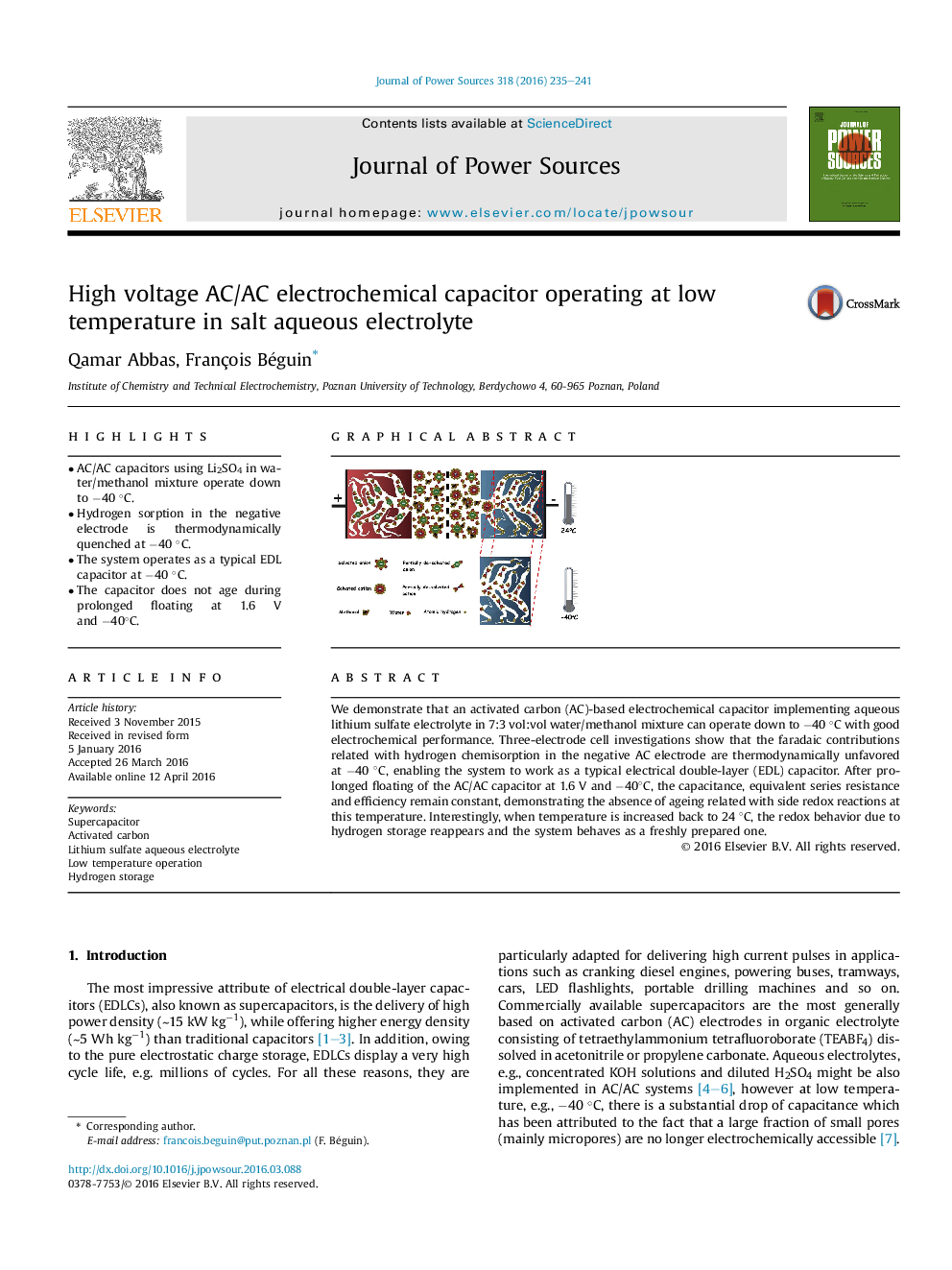
We demonstrate that an activated carbon (AC)-based electrochemical capacitor implementing aqueous lithium sulfate electrolyte in 7:3 vol:vol water/methanol mixture can operate down to −40 °C with good electrochemical performance. Three-electrode cell investigations show that the faradaic contributions related with hydrogen chemisorption in the negative AC electrode are thermodynamically unfavored at −40 °C, enabling the system to work as a typical electrical double-layer (EDL) capacitor. After prolonged floating of the AC/AC capacitor at 1.6 V and −40°C, the capacitance, equivalent series resistance and efficiency remain constant, demonstrating the absence of ageing related with side redox reactions at this temperature. Interestingly, when temperature is increased back to 24 °C, the redox behavior due to hydrogen storage reappears and the system behaves as a freshly prepared one.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide