| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1285302 | Journal of Power Sources | 2016 | 8 Pages |
•Electrochemical Na+-storage in hard carbons is explored using monolithic electrodes.•The Na+-insertion behaviors into hard carbons calcined at 800–3000 °C are compared.•The difference between Li+ and Na+ ion-trapping in nanovoids is demonstrated.•The effect of pore size on the initial irreversible capacity is discussed.
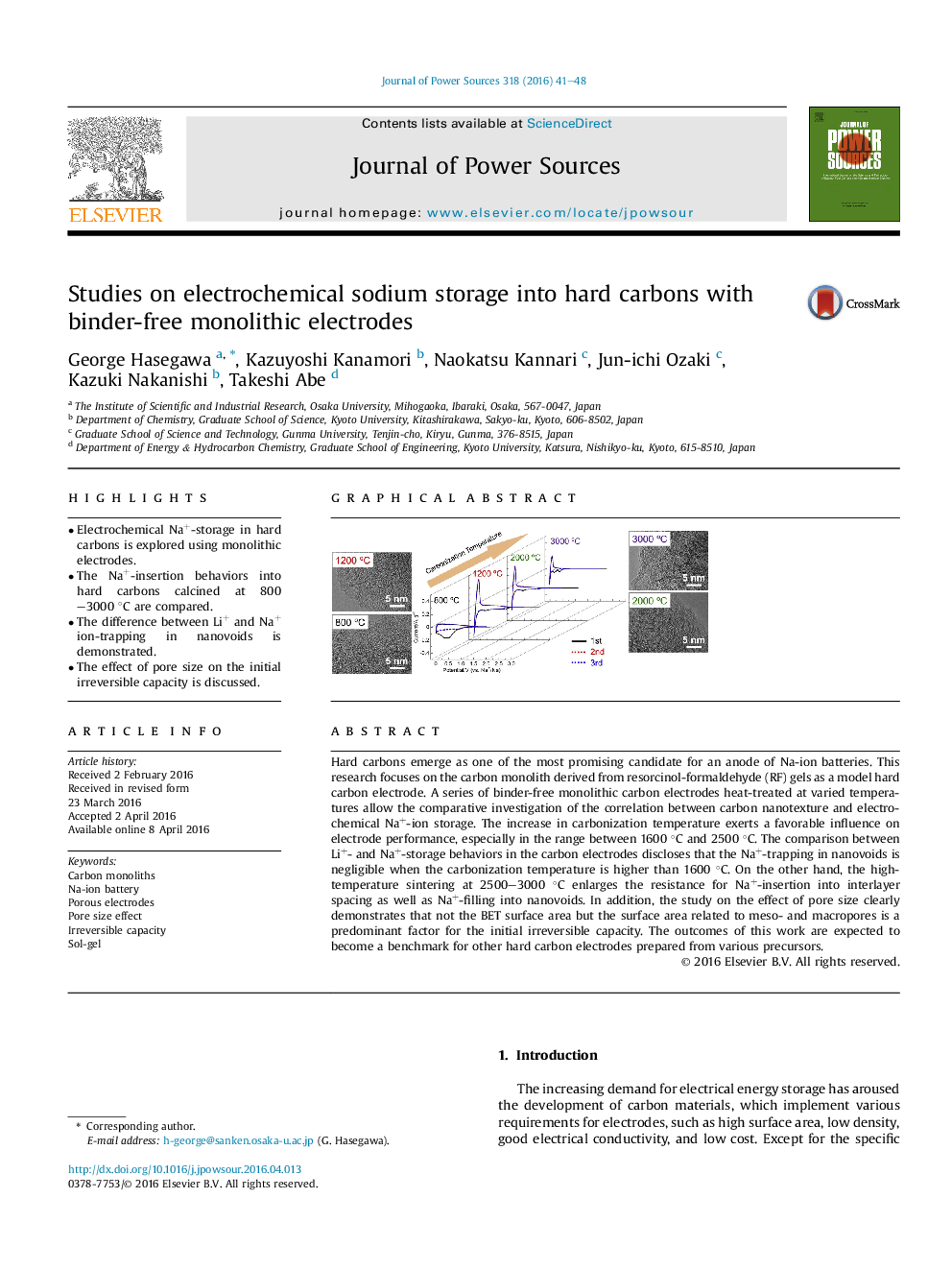
Hard carbons emerge as one of the most promising candidate for an anode of Na-ion batteries. This research focuses on the carbon monolith derived from resorcinol-formaldehyde (RF) gels as a model hard carbon electrode. A series of binder-free monolithic carbon electrodes heat-treated at varied temperatures allow the comparative investigation of the correlation between carbon nanotexture and electrochemical Na+-ion storage. The increase in carbonization temperature exerts a favorable influence on electrode performance, especially in the range between 1600 °C and 2500 °C. The comparison between Li+- and Na+-storage behaviors in the carbon electrodes discloses that the Na+-trapping in nanovoids is negligible when the carbonization temperature is higher than 1600 °C. On the other hand, the high-temperature sintering at 2500–3000 °C enlarges the resistance for Na+-insertion into interlayer spacing as well as Na+-filling into nanovoids. In addition, the study on the effect of pore size clearly demonstrates that not the BET surface area but the surface area related to meso- and macropores is a predominant factor for the initial irreversible capacity. The outcomes of this work are expected to become a benchmark for other hard carbon electrodes prepared from various precursors.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide