| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1285367 | Journal of Power Sources | 2016 | 9 Pages |
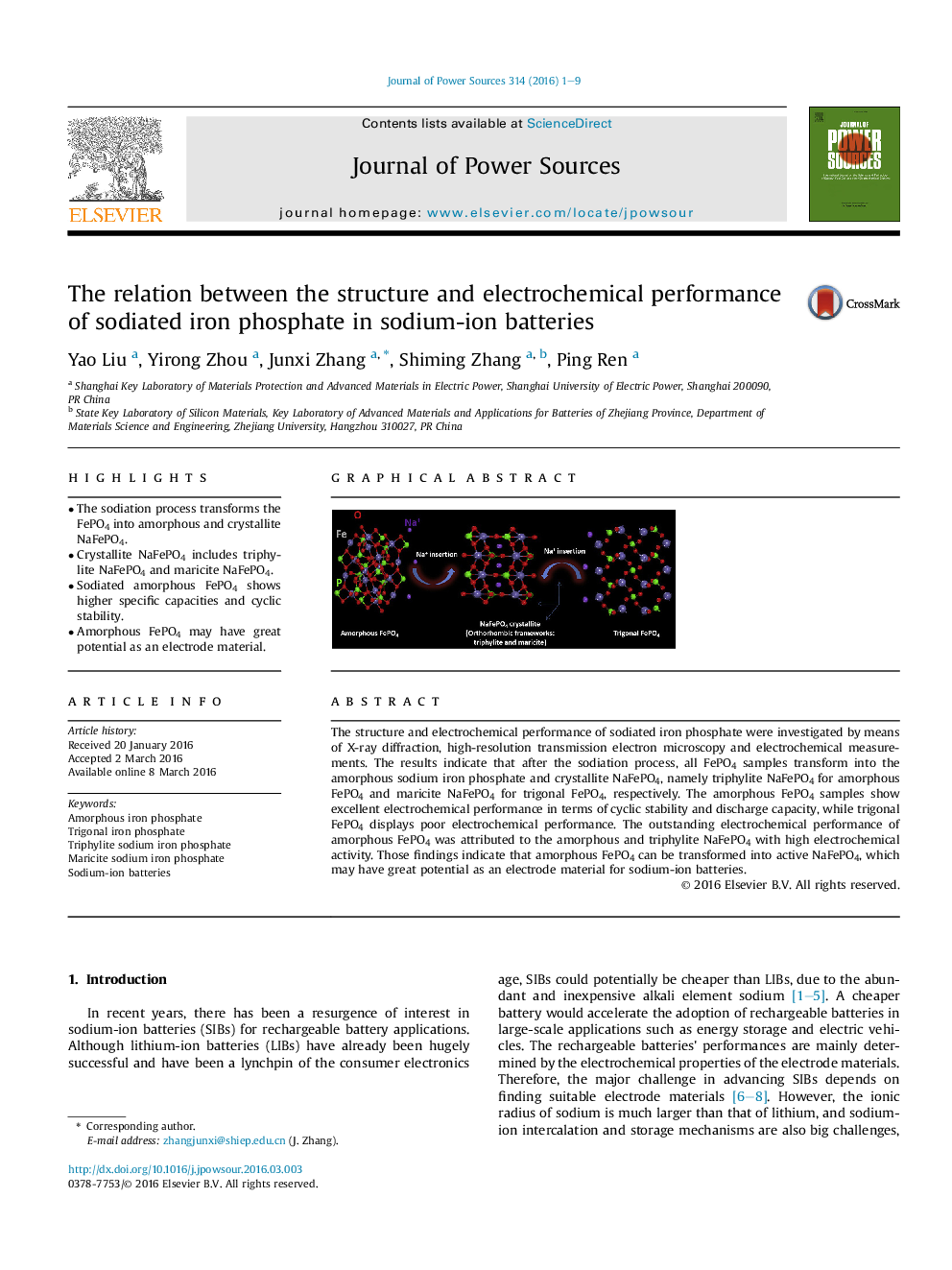
•The sodiation process transforms the FePO4 into amorphous and crystallite NaFePO4.•Crystallite NaFePO4 includes triphylite NaFePO4 and maricite NaFePO4.•Sodiated amorphous FePO4 shows higher specific capacities and cyclic stability.•Amorphous FePO4 may have great potential as an electrode material.
The structure and electrochemical performance of sodiated iron phosphate were investigated by means of X-ray diffraction, high-resolution transmission electron microscopy and electrochemical measurements. The results indicate that after the sodiation process, all FePO4 samples transform into the amorphous sodium iron phosphate and crystallite NaFePO4, namely triphylite NaFePO4 for amorphous FePO4 and maricite NaFePO4 for trigonal FePO4, respectively. The amorphous FePO4 samples show excellent electrochemical performance in terms of cyclic stability and discharge capacity, while trigonal FePO4 displays poor electrochemical performance. The outstanding electrochemical performance of amorphous FePO4 was attributed to the amorphous and triphylite NaFePO4 with high electrochemical activity. Those findings indicate that amorphous FePO4 can be transformed into active NaFePO4, which may have great potential as an electrode material for sodium-ion batteries.
Graphical abstractThe structural transformation of FePO4 nanoparticles during the sodiation process has been investigated by XRD and HRTEM. The results show that the sodiation process transforms the FePO4 into NaFePO4. Amorphous FePO4 partly transforms into highly active triphylite NaFePO4. Trigonal FePO4 partly transforms into maricite NaFePO4.Figure optionsDownload full-size imageDownload as PowerPoint slide