| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1285725 | Journal of Power Sources | 2016 | 9 Pages |
•The electrode shows unique 3D porous nanoarrays structure with a large surface area.•The electrode exhibits superior catalytic activity and stability for H2O2 reduction.•The reduction current density reaches 3.47 A mg−1 at the potential of 0.2 V.
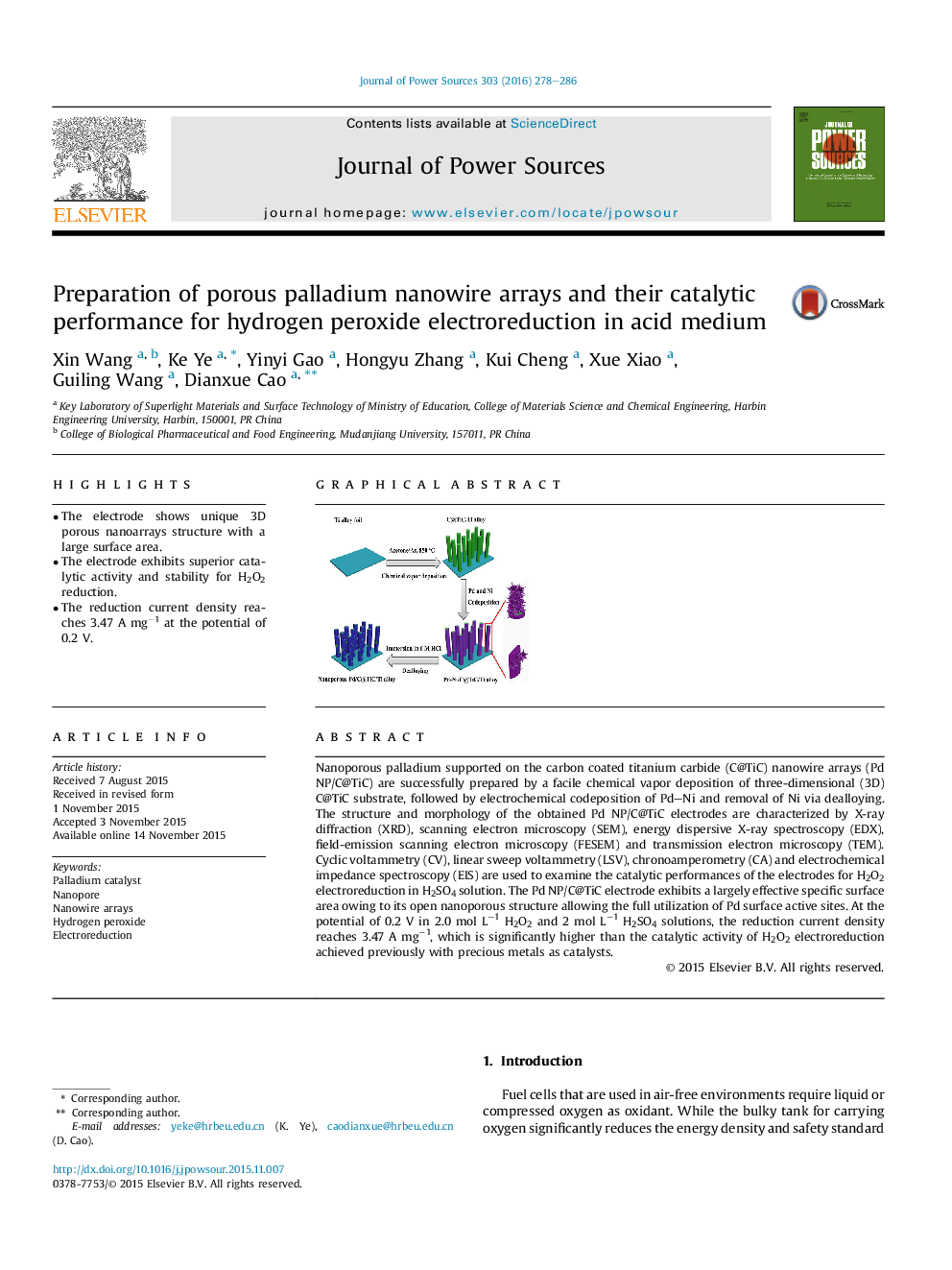
Nanoporous palladium supported on the carbon coated titanium carbide (C@TiC) nanowire arrays (Pd NP/C@TiC) are successfully prepared by a facile chemical vapor deposition of three-dimensional (3D) C@TiC substrate, followed by electrochemical codeposition of Pd–Ni and removal of Ni via dealloying. The structure and morphology of the obtained Pd NP/C@TiC electrodes are characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), field-emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). Cyclic voltammetry (CV), linear sweep voltammetry (LSV), chronoamperometry (CA) and electrochemical impedance spectroscopy (EIS) are used to examine the catalytic performances of the electrodes for H2O2 electroreduction in H2SO4 solution. The Pd NP/C@TiC electrode exhibits a largely effective specific surface area owing to its open nanoporous structure allowing the full utilization of Pd surface active sites. At the potential of 0.2 V in 2.0 mol L−1 H2O2 and 2 mol L−1 H2SO4 solutions, the reduction current density reaches 3.47 A mg−1, which is significantly higher than the catalytic activity of H2O2 electroreduction achieved previously with precious metals as catalysts.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide