| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1285729 | Journal of Power Sources | 2016 | 9 Pages |
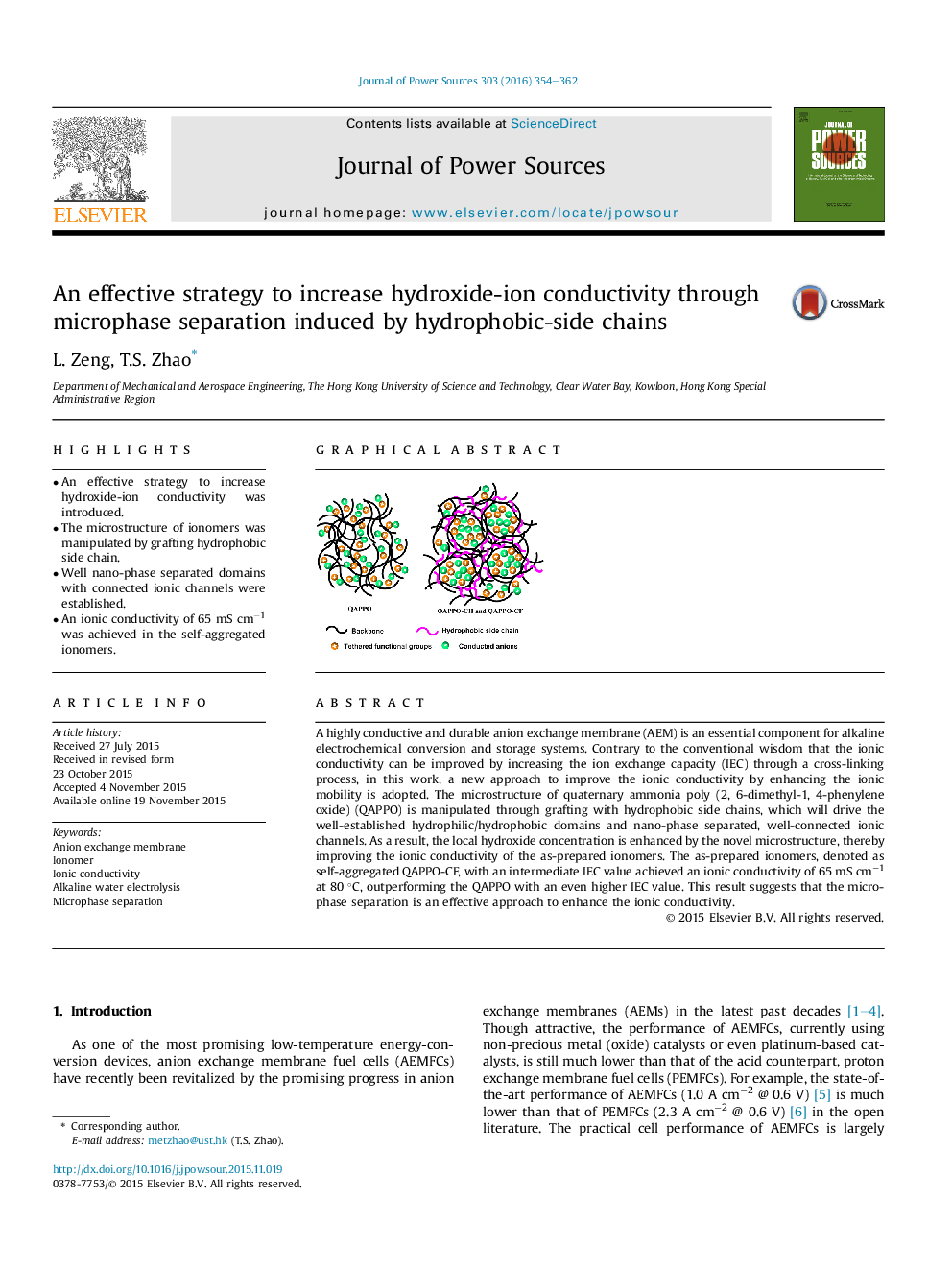
•An effective strategy to increase hydroxide-ion conductivity was introduced.•The microstructure of ionomers was manipulated by grafting hydrophobic side chain.•Well nano-phase separated domains with connected ionic channels were established.•An ionic conductivity of 65 mS cm−1 was achieved in the self-aggregated ionomers.
A highly conductive and durable anion exchange membrane (AEM) is an essential component for alkaline electrochemical conversion and storage systems. Contrary to the conventional wisdom that the ionic conductivity can be improved by increasing the ion exchange capacity (IEC) through a cross-linking process, in this work, a new approach to improve the ionic conductivity by enhancing the ionic mobility is adopted. The microstructure of quaternary ammonia poly (2, 6-dimethyl-1, 4-phenylene oxide) (QAPPO) is manipulated through grafting with hydrophobic side chains, which will drive the well-established hydrophilic/hydrophobic domains and nano-phase separated, well-connected ionic channels. As a result, the local hydroxide concentration is enhanced by the novel microstructure, thereby improving the ionic conductivity of the as-prepared ionomers. The as-prepared ionomers, denoted as self-aggregated QAPPO-CF, with an intermediate IEC value achieved an ionic conductivity of 65 mS cm−1 at 80 °C, outperforming the QAPPO with an even higher IEC value. This result suggests that the microphase separation is an effective approach to enhance the ionic conductivity.
Graphical abstractThe well-established hydrophilic/hydrophobic domains and nano-phase separated, well-connected ionic channels were manipulated through grafting the hydrophobic side chains on the backbones of quaternary ammonia poly (2, 6-dimethyl-1, 4-phenylene oxide). The ionic conductivity of self-aggregated ionomers with an intermediate IEC value outperformed the traditional ionomers with an even high IEC value.Figure optionsDownload full-size imageDownload as PowerPoint slide