| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1286571 | Journal of Power Sources | 2014 | 9 Pages |
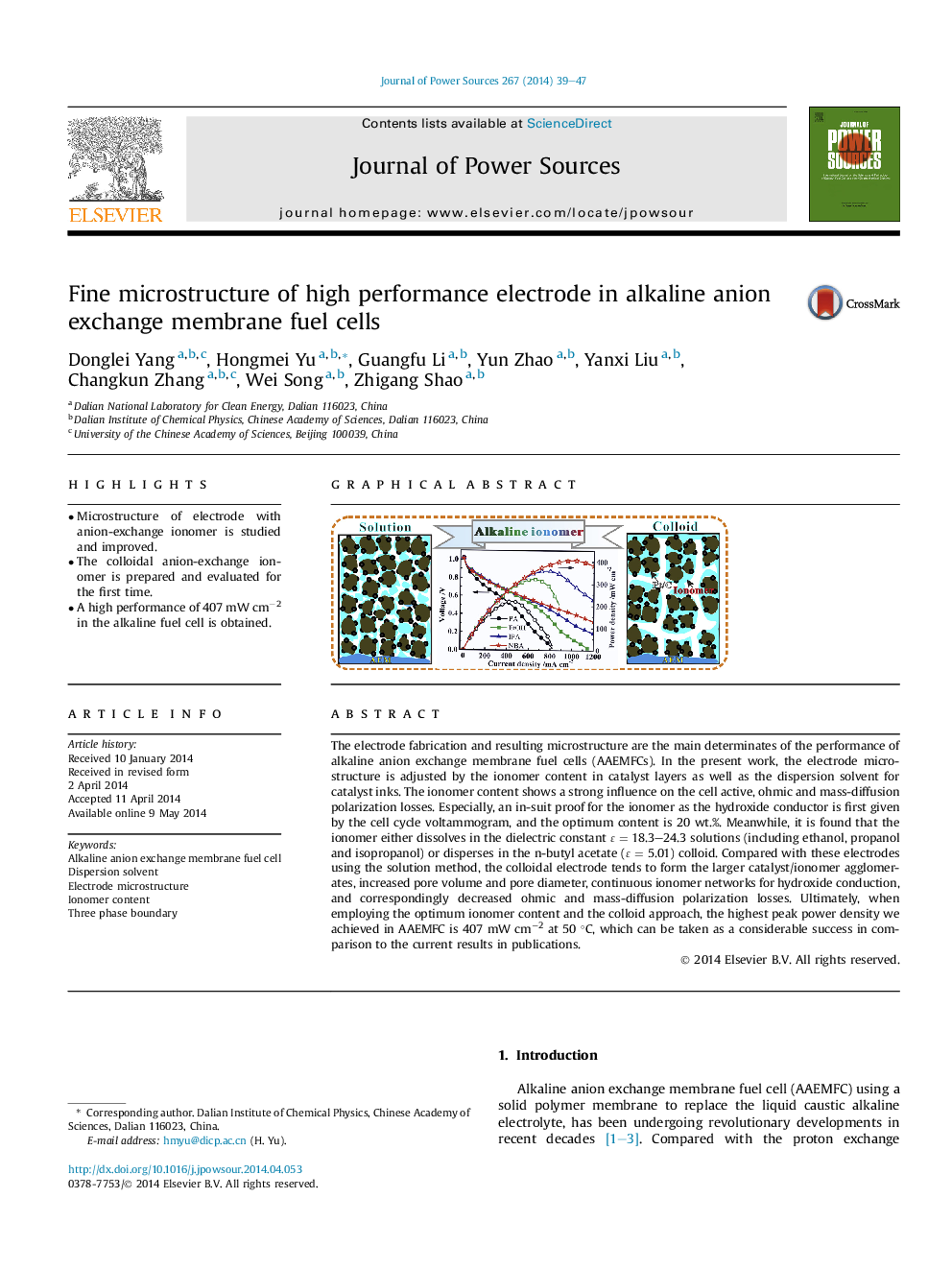
•Microstructure of electrode with anion-exchange ionomer is studied and improved.•The colloidal anion-exchange ionomer is prepared and evaluated for the first time.•A high performance of 407 mW cm−2 in the alkaline fuel cell is obtained.
The electrode fabrication and resulting microstructure are the main determinates of the performance of alkaline anion exchange membrane fuel cells (AAEMFCs). In the present work, the electrode microstructure is adjusted by the ionomer content in catalyst layers as well as the dispersion solvent for catalyst inks. The ionomer content shows a strong influence on the cell active, ohmic and mass-diffusion polarization losses. Especially, an in-suit proof for the ionomer as the hydroxide conductor is first given by the cell cycle voltammogram, and the optimum content is 20 wt.%. Meanwhile, it is found that the ionomer either dissolves in the dielectric constant ɛ = 18.3–24.3 solutions (including ethanol, propanol and isopropanol) or disperses in the n-butyl acetate (ɛ = 5.01) colloid. Compared with these electrodes using the solution method, the colloidal electrode tends to form the larger catalyst/ionomer agglomerates, increased pore volume and pore diameter, continuous ionomer networks for hydroxide conduction, and correspondingly decreased ohmic and mass-diffusion polarization losses. Ultimately, when employing the optimum ionomer content and the colloid approach, the highest peak power density we achieved in AAEMFC is 407 mW cm−2 at 50 °C, which can be taken as a considerable success in comparison to the current results in publications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide