| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1286795 | Journal of Power Sources | 2015 | 8 Pages |
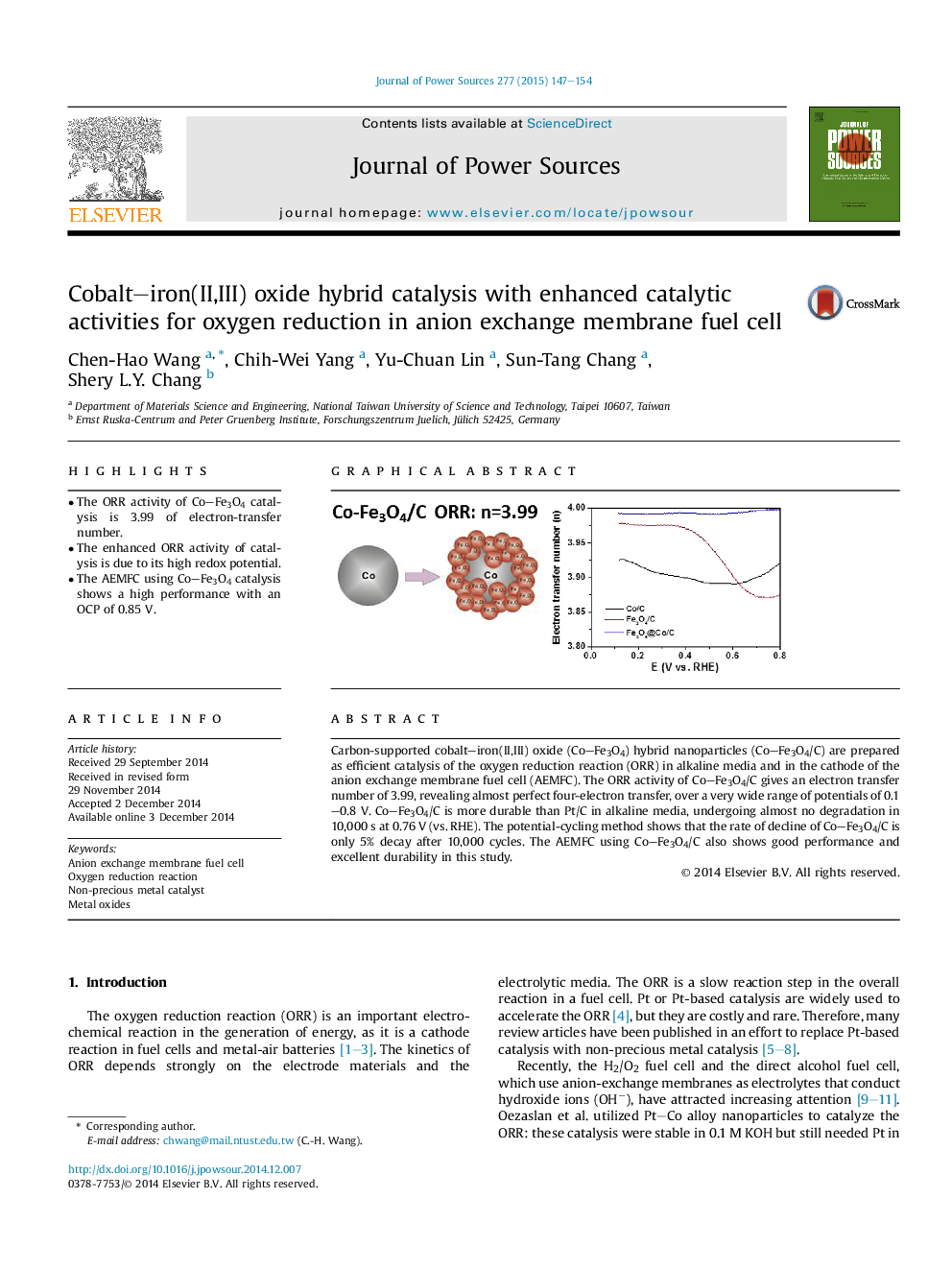
•The ORR activity of Co–Fe3O4 catalysis is 3.99 of electron-transfer number.•The enhanced ORR activity of catalysis is due to its high redox potential.•The AEMFC using Co–Fe3O4 catalysis shows a high performance with an OCP of 0.85 V.
Carbon-supported cobalt–iron(II,III) oxide (Co–Fe3O4) hybrid nanoparticles (Co–Fe3O4/C) are prepared as efficient catalysis of the oxygen reduction reaction (ORR) in alkaline media and in the cathode of the anion exchange membrane fuel cell (AEMFC). The ORR activity of Co–Fe3O4/C gives an electron transfer number of 3.99, revealing almost perfect four-electron transfer, over a very wide range of potentials of 0.1–0.8 V. Co–Fe3O4/C is more durable than Pt/C in alkaline media, undergoing almost no degradation in 10,000 s at 0.76 V (vs. RHE). The potential-cycling method shows that the rate of decline of Co–Fe3O4/C is only 5% decay after 10,000 cycles. The AEMFC using Co–Fe3O4/C also shows good performance and excellent durability in this study.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide