| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1287108 | Journal of Power Sources | 2014 | 9 Pages |
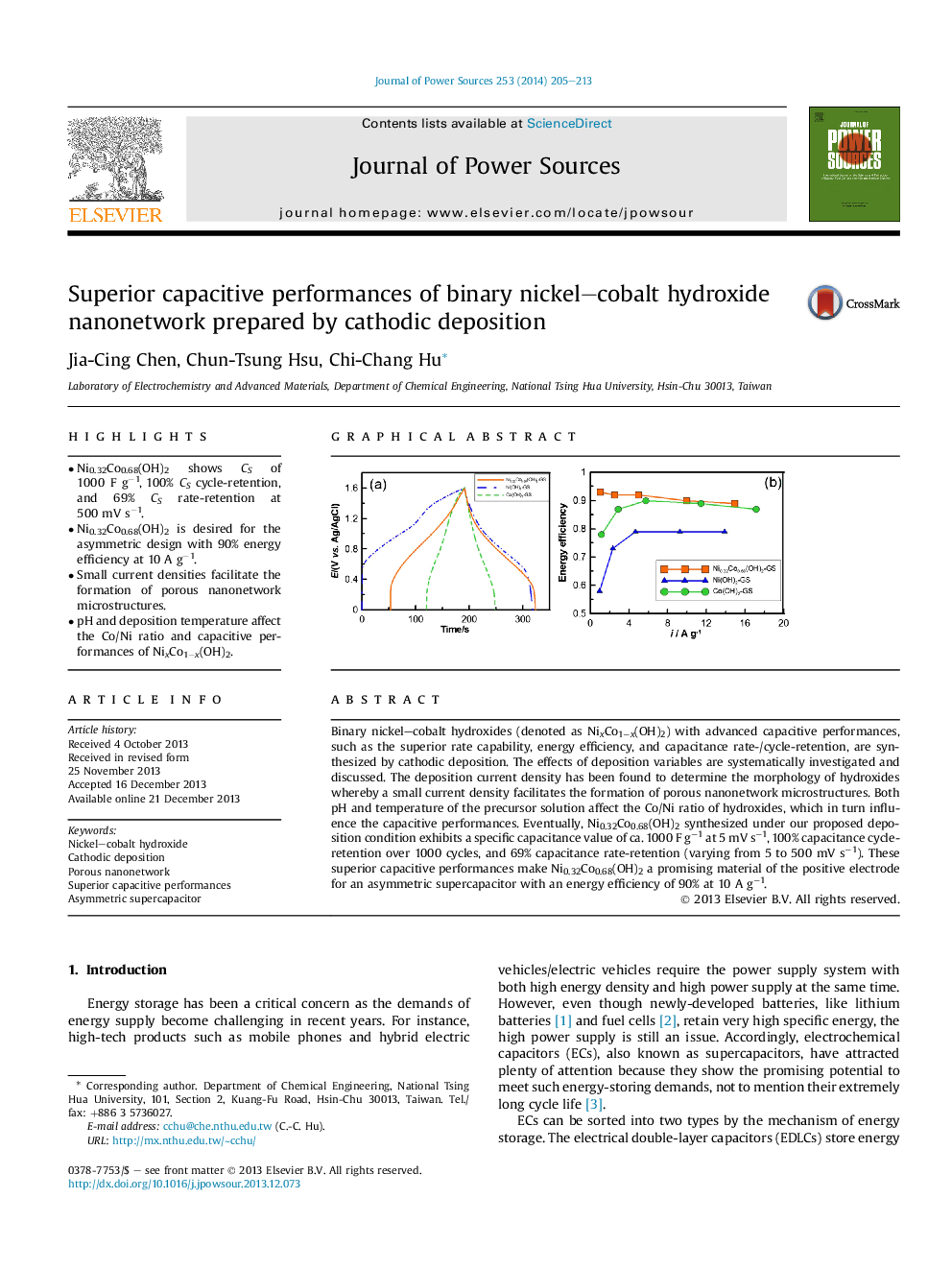
•Ni0.32Co0.68(OH)2 shows CS of 1000 F g−1, 100% CS cycle-retention, and 69% CS rate-retention at 500 mV s−1.•Ni0.32Co0.68(OH)2 is desired for the asymmetric design with 90% energy efficiency at 10 A g−1.•Small current densities facilitate the formation of porous nanonetwork microstructures.•pH and deposition temperature affect the Co/Ni ratio and capacitive performances of NixCo1−x(OH)2.
Binary nickel–cobalt hydroxides (denoted as NixCo1−x(OH)2) with advanced capacitive performances, such as the superior rate capability, energy efficiency, and capacitance rate-/cycle-retention, are synthesized by cathodic deposition. The effects of deposition variables are systematically investigated and discussed. The deposition current density has been found to determine the morphology of hydroxides whereby a small current density facilitates the formation of porous nanonetwork microstructures. Both pH and temperature of the precursor solution affect the Co/Ni ratio of hydroxides, which in turn influence the capacitive performances. Eventually, Ni0.32Co0.68(OH)2 synthesized under our proposed deposition condition exhibits a specific capacitance value of ca. 1000 F g−1 at 5 mV s−1, 100% capacitance cycle-retention over 1000 cycles, and 69% capacitance rate-retention (varying from 5 to 500 mV s−1). These superior capacitive performances make Ni0.32Co0.68(OH)2 a promising material of the positive electrode for an asymmetric supercapacitor with an energy efficiency of 90% at 10 A g−1.
Graphical abstractThe Ni0.32Co0.68(OH)2–graphene asymmetric supercapacitor exhibits superior capacitive performances of a high energy efficiency, high specific energy, excellent reversibility, and outstanding cycle stability. Its specific power and energy simultaneously reaching 23.9 kW kg−1 and 24 Wh kg−1, respectively.Figure optionsDownload full-size imageDownload as PowerPoint slide