| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1287520 | Journal of Power Sources | 2013 | 6 Pages |
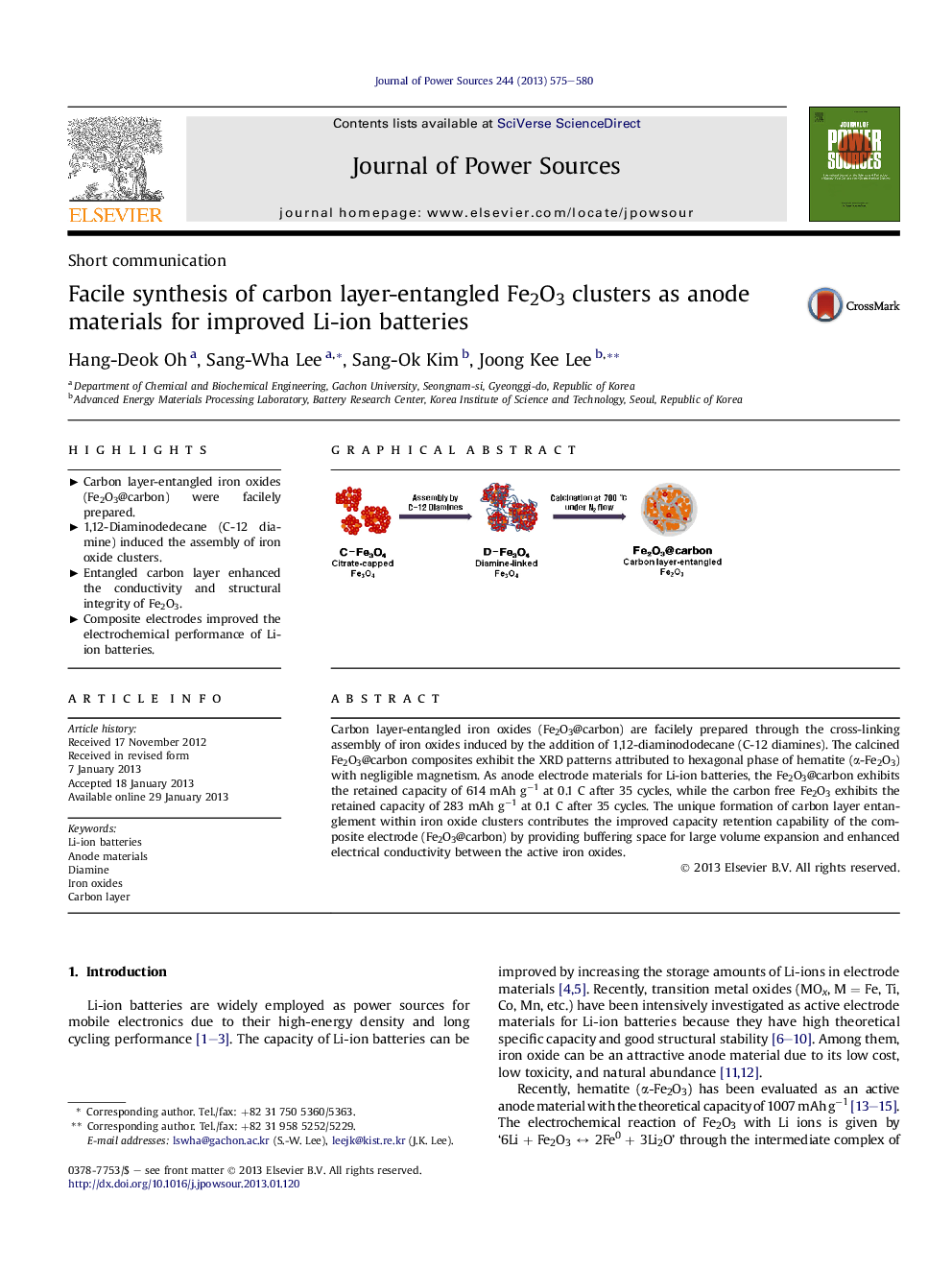
Carbon layer-entangled iron oxides (Fe2O3@carbon) are facilely prepared through the cross-linking assembly of iron oxides induced by the addition of 1,12-diaminododecane (C-12 diamines). The calcined Fe2O3@carbon composites exhibit the XRD patterns attributed to hexagonal phase of hematite (α-Fe2O3) with negligible magnetism. As anode electrode materials for Li-ion batteries, the Fe2O3@carbon exhibits the retained capacity of 614 mAh g−1 at 0.1 C after 35 cycles, while the carbon free Fe2O3 exhibits the retained capacity of 283 mAh g−1 at 0.1 C after 35 cycles. The unique formation of carbon layer entanglement within iron oxide clusters contributes the improved capacity retention capability of the composite electrode (Fe2O3@carbon) by providing buffering space for large volume expansion and enhanced electrical conductivity between the active iron oxides.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Carbon layer-entangled iron oxides (Fe2O3@carbon) were facilely prepared. ► 1,12-Diaminodedecane (C-12 diamine) induced the assembly of iron oxide clusters. ► Entangled carbon layer enhanced the conductivity and structural integrity of Fe2O3. ► Composite electrodes improved the electrochemical performance of Li-ion batteries.