| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1287954 | Journal of Power Sources | 2013 | 5 Pages |
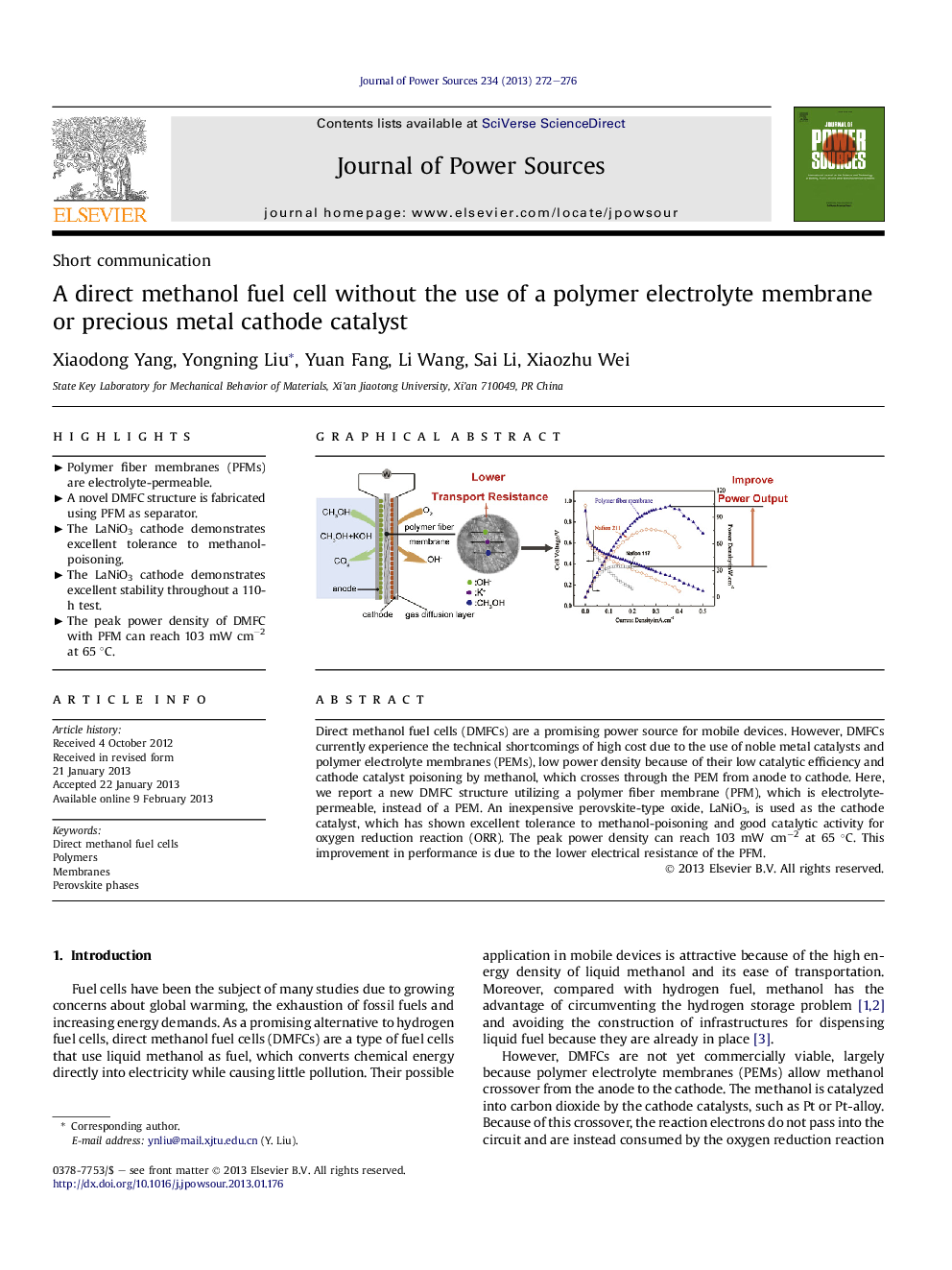
Direct methanol fuel cells (DMFCs) are a promising power source for mobile devices. However, DMFCs currently experience the technical shortcomings of high cost due to the use of noble metal catalysts and polymer electrolyte membranes (PEMs), low power density because of their low catalytic efficiency and cathode catalyst poisoning by methanol, which crosses through the PEM from anode to cathode. Here, we report a new DMFC structure utilizing a polymer fiber membrane (PFM), which is electrolyte-permeable, instead of a PEM. An inexpensive perovskite-type oxide, LaNiO3, is used as the cathode catalyst, which has shown excellent tolerance to methanol-poisoning and good catalytic activity for oxygen reduction reaction (ORR). The peak power density can reach 103 mW cm−2 at 65 °C. This improvement in performance is due to the lower electrical resistance of the PFM.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Polymer fiber membranes (PFMs) are electrolyte-permeable. ► A novel DMFC structure is fabricated using PFM as separator. ► The LaNiO3 cathode demonstrates excellent tolerance to methanol-poisoning. ► The LaNiO3 cathode demonstrates excellent stability throughout a 110-h test. ► The peak power density of DMFC with PFM can reach 103 mW cm−2 at 65 °C.