| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1292365 | Journal of Power Sources | 2016 | 9 Pages |
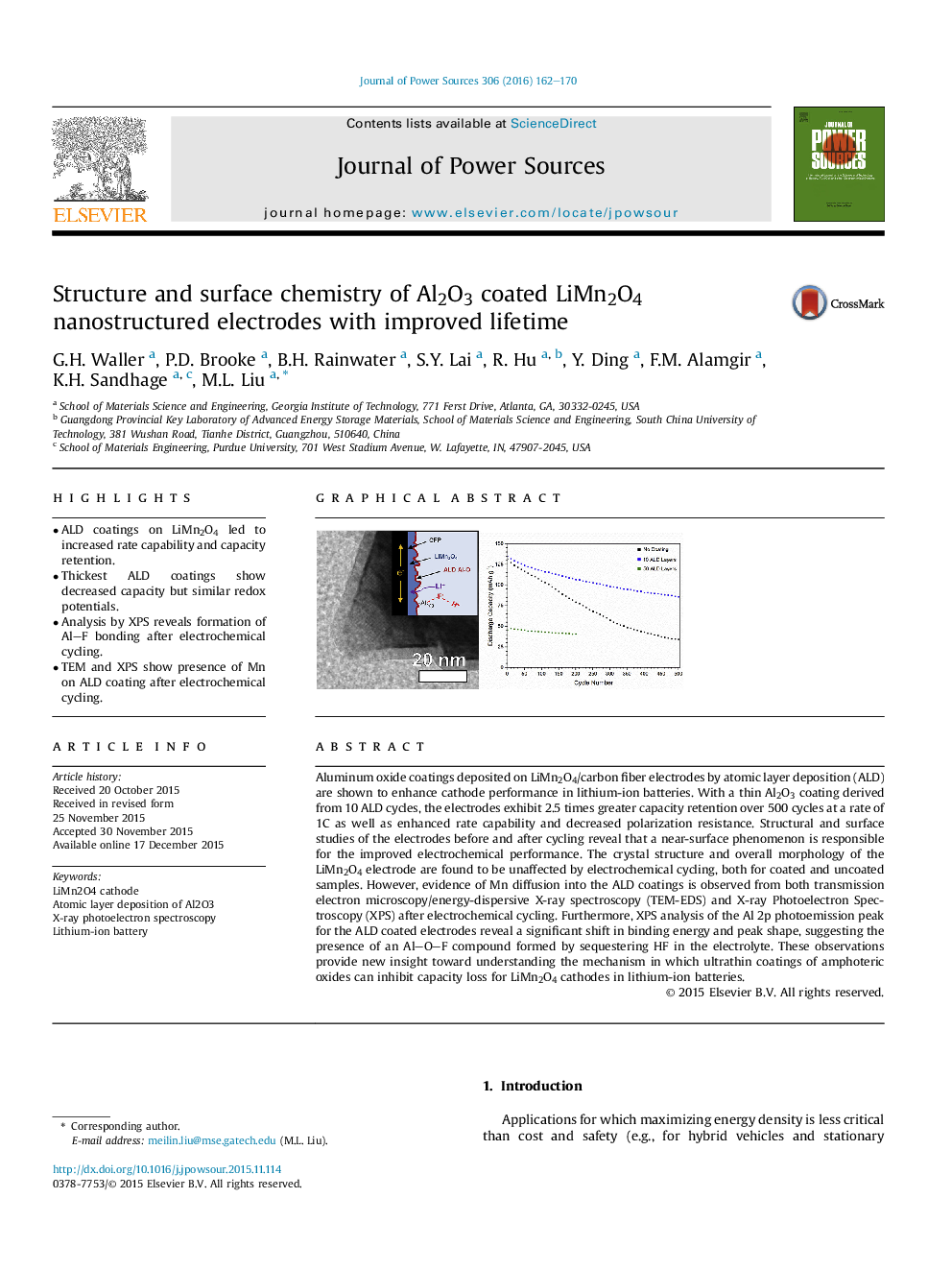
•ALD coatings on LiMn2O4 led to increased rate capability and capacity retention.•Thickest ALD coatings show decreased capacity but similar redox potentials.•Analysis by XPS reveals formation of Al–F bonding after electrochemical cycling.•TEM and XPS show presence of Mn on ALD coating after electrochemical cycling.
Aluminum oxide coatings deposited on LiMn2O4/carbon fiber electrodes by atomic layer deposition (ALD) are shown to enhance cathode performance in lithium-ion batteries. With a thin Al2O3 coating derived from 10 ALD cycles, the electrodes exhibit 2.5 times greater capacity retention over 500 cycles at a rate of 1C as well as enhanced rate capability and decreased polarization resistance. Structural and surface studies of the electrodes before and after cycling reveal that a near-surface phenomenon is responsible for the improved electrochemical performance. The crystal structure and overall morphology of the LiMn2O4 electrode are found to be unaffected by electrochemical cycling, both for coated and uncoated samples. However, evidence of Mn diffusion into the ALD coatings is observed from both transmission electron microscopy/energy-dispersive X-ray spectroscopy (TEM-EDS) and X-ray Photoelectron Spectroscopy (XPS) after electrochemical cycling. Furthermore, XPS analysis of the Al 2p photoemission peak for the ALD coated electrodes reveal a significant shift in binding energy and peak shape, suggesting the presence of an Al–O–F compound formed by sequestering HF in the electrolyte. These observations provide new insight toward understanding the mechanism in which ultrathin coatings of amphoteric oxides can inhibit capacity loss for LiMn2O4 cathodes in lithium-ion batteries.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide