| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1292854 | Journal of Power Sources | 2015 | 9 Pages |
•Li-rich cathode is synthesized by co-precipitation method.•Rieltveld refinement reveals enlarged interlayer spacing of F dop cathode.•F doping retards the undesired layered-to-spinel phase transition.•F doped materials show significantly increased cycling stability and rate performance.
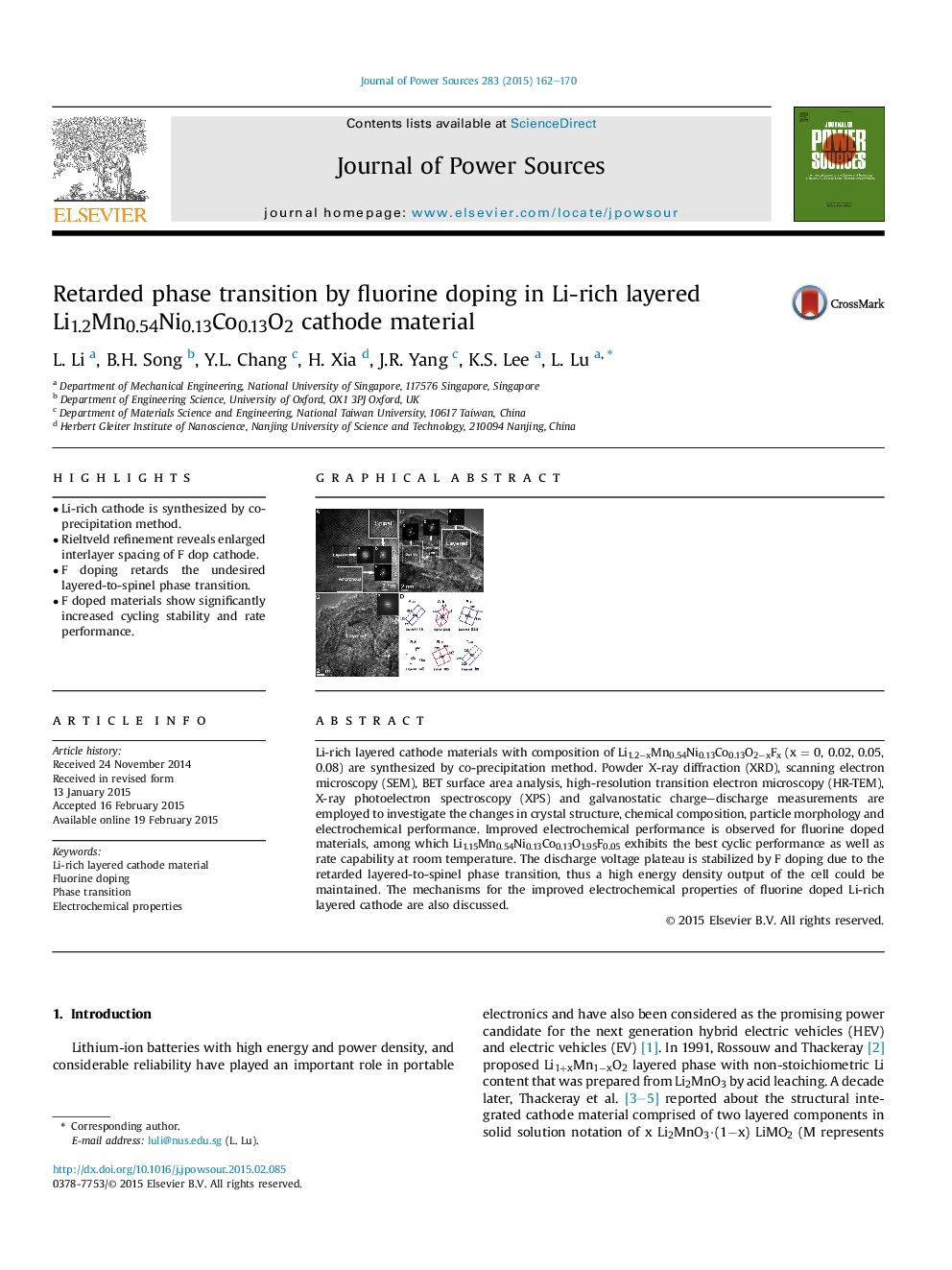
Li-rich layered cathode materials with composition of Li1.2−xMn0.54Ni0.13Co0.13O2−xFx (x = 0, 0.02, 0.05, 0.08) are synthesized by co-precipitation method. Powder X-ray diffraction (XRD), scanning electron microscopy (SEM), BET surface area analysis, high-resolution transition electron microscopy (HR-TEM), X-ray photoelectron spectroscopy (XPS) and galvanostatic charge–discharge measurements are employed to investigate the changes in crystal structure, chemical composition, particle morphology and electrochemical performance. Improved electrochemical performance is observed for fluorine doped materials, among which Li1.15Mn0.54Ni0.13Co0.13O1.95F0.05 exhibits the best cyclic performance as well as rate capability at room temperature. The discharge voltage plateau is stabilized by F doping due to the retarded layered-to-spinel phase transition, thus a high energy density output of the cell could be maintained. The mechanisms for the improved electrochemical properties of fluorine doped Li-rich layered cathode are also discussed.
Graphical abstractF-stabilized layer structure.Figure optionsDownload full-size imageDownload as PowerPoint slide