| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1292860 | Journal of Power Sources | 2015 | 11 Pages |
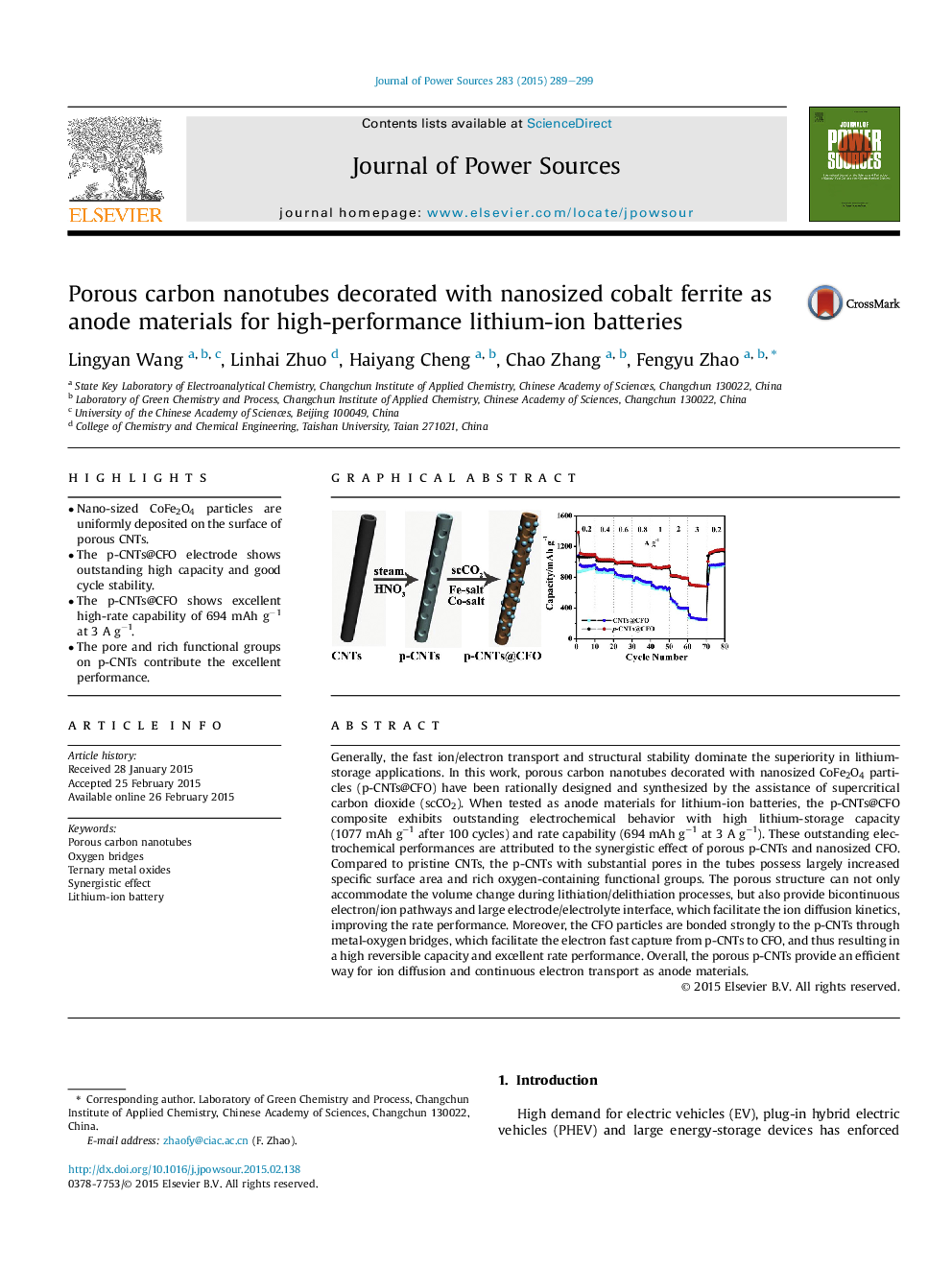
•Nano-sized CoFe2O4 particles are uniformly deposited on the surface of porous CNTs.•The p-CNTs@CFO electrode shows outstanding high capacity and good cycle stability.•The p-CNTs@CFO shows excellent high-rate capability of 694 mAh g−1 at 3 A g−1.•The pore and rich functional groups on p-CNTs contribute the excellent performance.
Generally, the fast ion/electron transport and structural stability dominate the superiority in lithium-storage applications. In this work, porous carbon nanotubes decorated with nanosized CoFe2O4 particles (p-CNTs@CFO) have been rationally designed and synthesized by the assistance of supercritical carbon dioxide (scCO2). When tested as anode materials for lithium-ion batteries, the p-CNTs@CFO composite exhibits outstanding electrochemical behavior with high lithium-storage capacity (1077 mAh g−1 after 100 cycles) and rate capability (694 mAh g−1 at 3 A g−1). These outstanding electrochemical performances are attributed to the synergistic effect of porous p-CNTs and nanosized CFO. Compared to pristine CNTs, the p-CNTs with substantial pores in the tubes possess largely increased specific surface area and rich oxygen-containing functional groups. The porous structure can not only accommodate the volume change during lithiation/delithiation processes, but also provide bicontinuous electron/ion pathways and large electrode/electrolyte interface, which facilitate the ion diffusion kinetics, improving the rate performance. Moreover, the CFO particles are bonded strongly to the p-CNTs through metal-oxygen bridges, which facilitate the electron fast capture from p-CNTs to CFO, and thus resulting in a high reversible capacity and excellent rate performance. Overall, the porous p-CNTs provide an efficient way for ion diffusion and continuous electron transport as anode materials.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide