| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1362531 | Bioorganic & Medicinal Chemistry Letters | 2010 | 4 Pages |
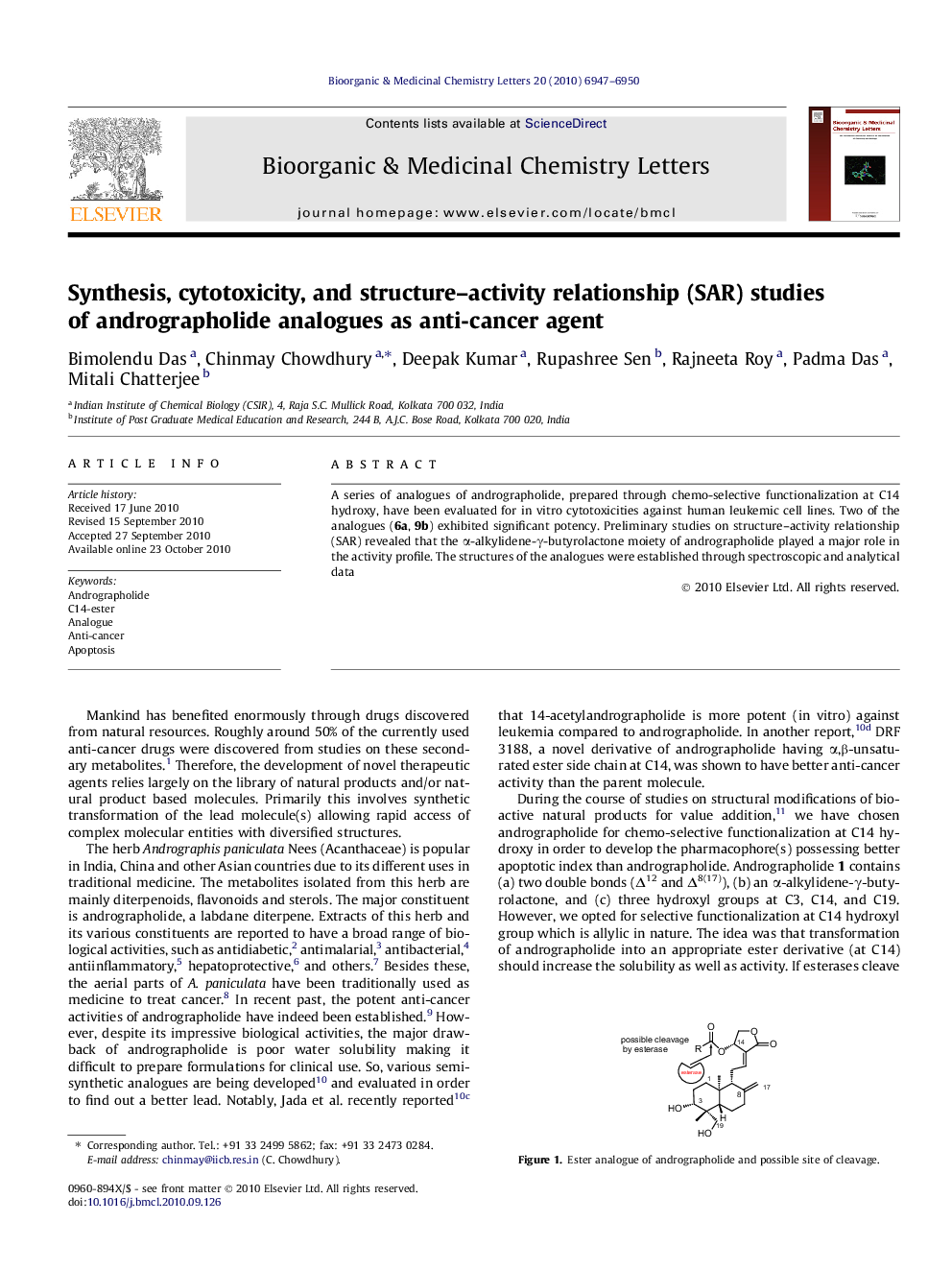
A series of analogues of andrographolide, prepared through chemo-selective functionalization at C14 hydroxy, have been evaluated for in vitro cytotoxicities against human leukemic cell lines. Two of the analogues (6a, 9b) exhibited significant potency. Preliminary studies on structure–activity relationship (SAR) revealed that the α-alkylidene-γ-butyrolactone moiety of andrographolide played a major role in the activity profile. The structures of the analogues were established through spectroscopic and analytical data
Graphical abstractA series of analogues of andrographolide, prepared through chemo-selective functionalization at C14 hydroxy, have been evaluated for in vitro cytotoxicities against human leukemic cell lines. Two of the analogues (6a, 9b) exhibited significant potency. Preliminary studies on structure–activity relationship (SAR) revealed that the α-alkylidene-γ-butyrolactone moiety of andrographolide played a major role in the activity profile. The structures of the analogues were established through spectroscopic and analytical data.Figure optionsDownload full-size imageDownload as PowerPoint slide