| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1363392 | Bioorganic & Medicinal Chemistry Letters | 2010 | 4 Pages |
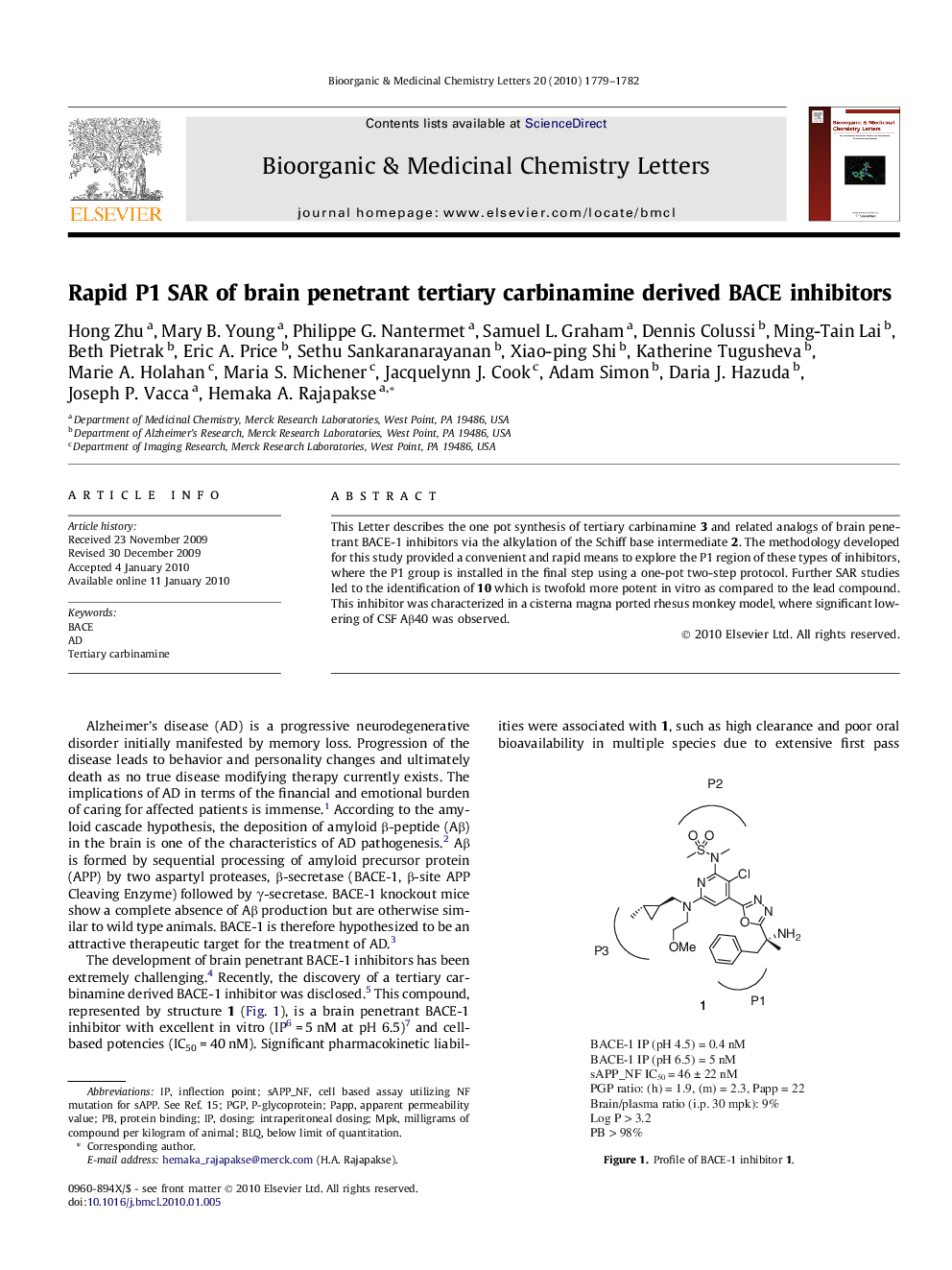
This Letter describes the one pot synthesis of tertiary carbinamine 3 and related analogs of brain penetrant BACE-1 inhibitors via the alkylation of the Schiff base intermediate 2. The methodology developed for this study provided a convenient and rapid means to explore the P1 region of these types of inhibitors, where the P1 group is installed in the final step using a one-pot two-step protocol. Further SAR studies led to the identification of 10 which is twofold more potent in vitro as compared to the lead compound. This inhibitor was characterized in a cisterna magna ported rhesus monkey model, where significant lowering of CSF Aβ40 was observed.
Graphical abstractThis Letter describes the one pot synthesis of tertiary carbinamine 3 and related analogs of brain penetrant BACE-1 inhibitors via the alkylation of the Schiff base intermediate 2. Extensive SAR studies led to the identification of a potent BACE inhibitor which is twofold more potent in vitro compared to the lead compound. Significant lowering of CSF Aβ40 was observed in a cisterna magna ported rhesus monkey model with the administration of compound 3.Figure optionsDownload full-size imageDownload as PowerPoint slide