| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1364585 | Bioorganic & Medicinal Chemistry Letters | 2008 | 5 Pages |
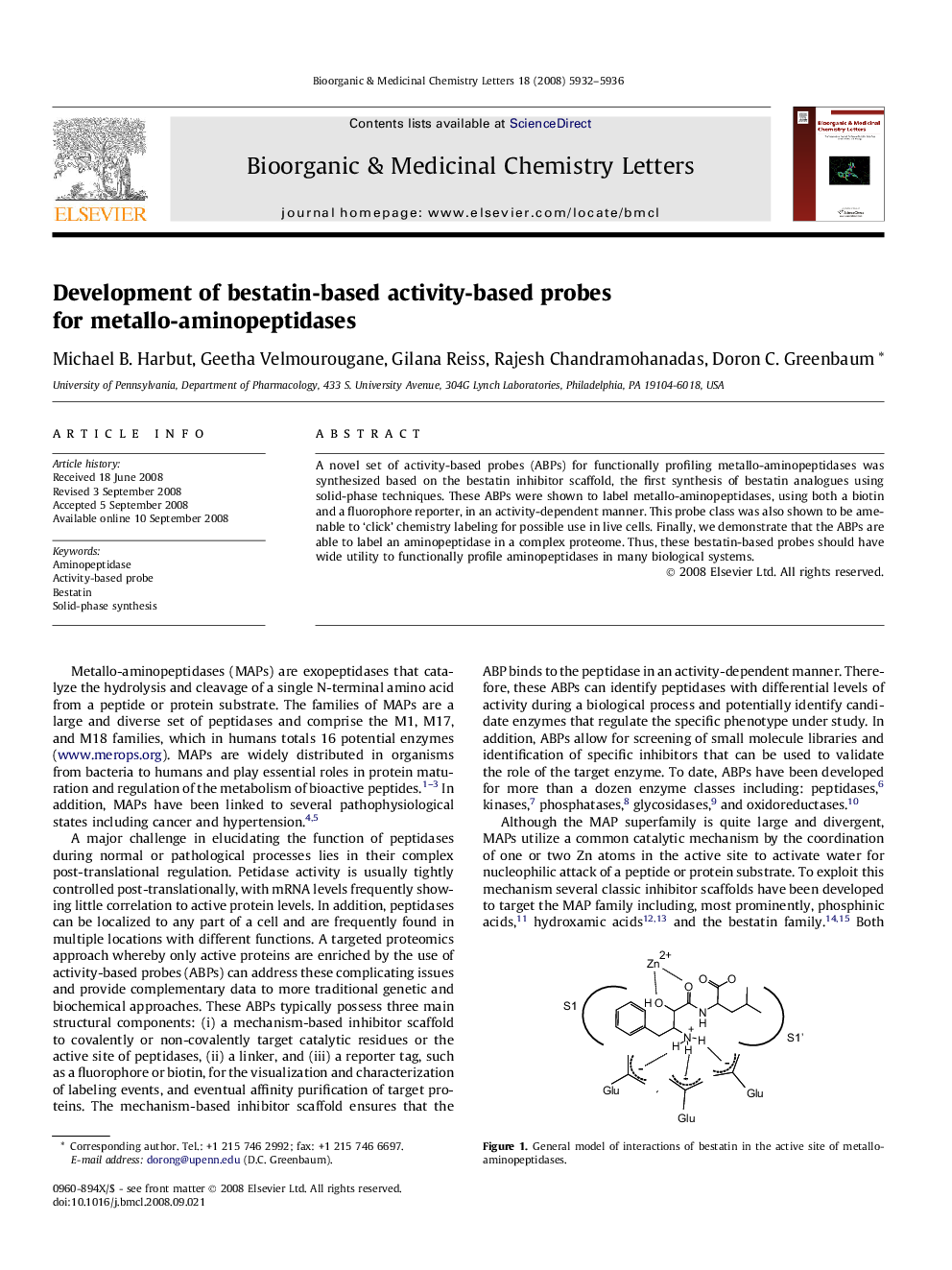
A novel set of activity-based probes (ABPs) for functionally profiling metallo-aminopeptidases was synthesized based on the bestatin inhibitor scaffold, the first synthesis of bestatin analogues using solid-phase techniques. These ABPs were shown to label metallo-aminopeptidases, using both a biotin and a fluorophore reporter, in an activity-dependent manner. This probe class was also shown to be amenable to ‘click’ chemistry labeling for possible use in live cells. Finally, we demonstrate that the ABPs are able to label an aminopeptidase in a complex proteome. Thus, these bestatin-based probes should have wide utility to functionally profile aminopeptidases in many biological systems.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide