| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1366537 | Bioorganic & Medicinal Chemistry Letters | 2007 | 4 Pages |
Abstract
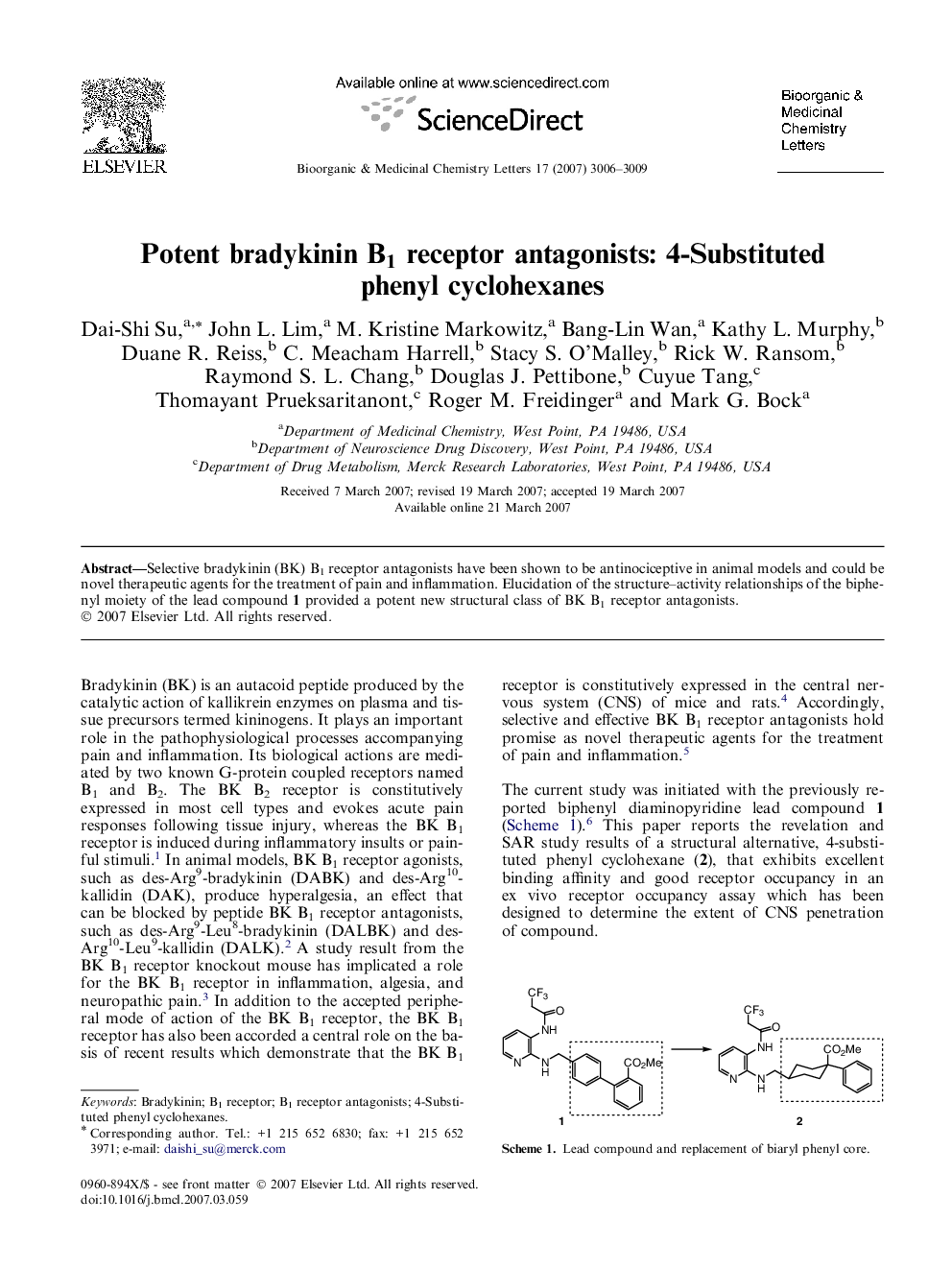
Selective bradykinin (BK) B1 receptor antagonists have been shown to be antinociceptive in animal models and could be novel therapeutic agents for the treatment of pain and inflammation. Elucidation of the structure–activity relationships of the biphenyl moiety of the lead compound 1 provided a potent new structural class of BK B1 receptor antagonists.
Graphical abstract4-Substituted phenyl cyclohexanes were identified as alternative isosteres for the biphenyl moiety of the lead compound 1. The syntheses, SAR optimization, and pharmacokinetic profiles of these compounds are described.Figure optionsDownload full-size imageDownload as PowerPoint slide
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Dai-Shi Su, John L. Lim, M. Kristine Markowitz, Bang-Lin Wan, Kathy L. Murphy, Duane R. Reiss, C. Meacham Harrell, Stacy S. O’Malley, Rick W. Ransom, Raymond S.L. Chang, Douglas J. Pettibone, Cuyue Tang, Thomayant Prueksaritanont, Roger M. Freidinger,