| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367162 | Bioorganic & Medicinal Chemistry Letters | 2006 | 5 Pages |
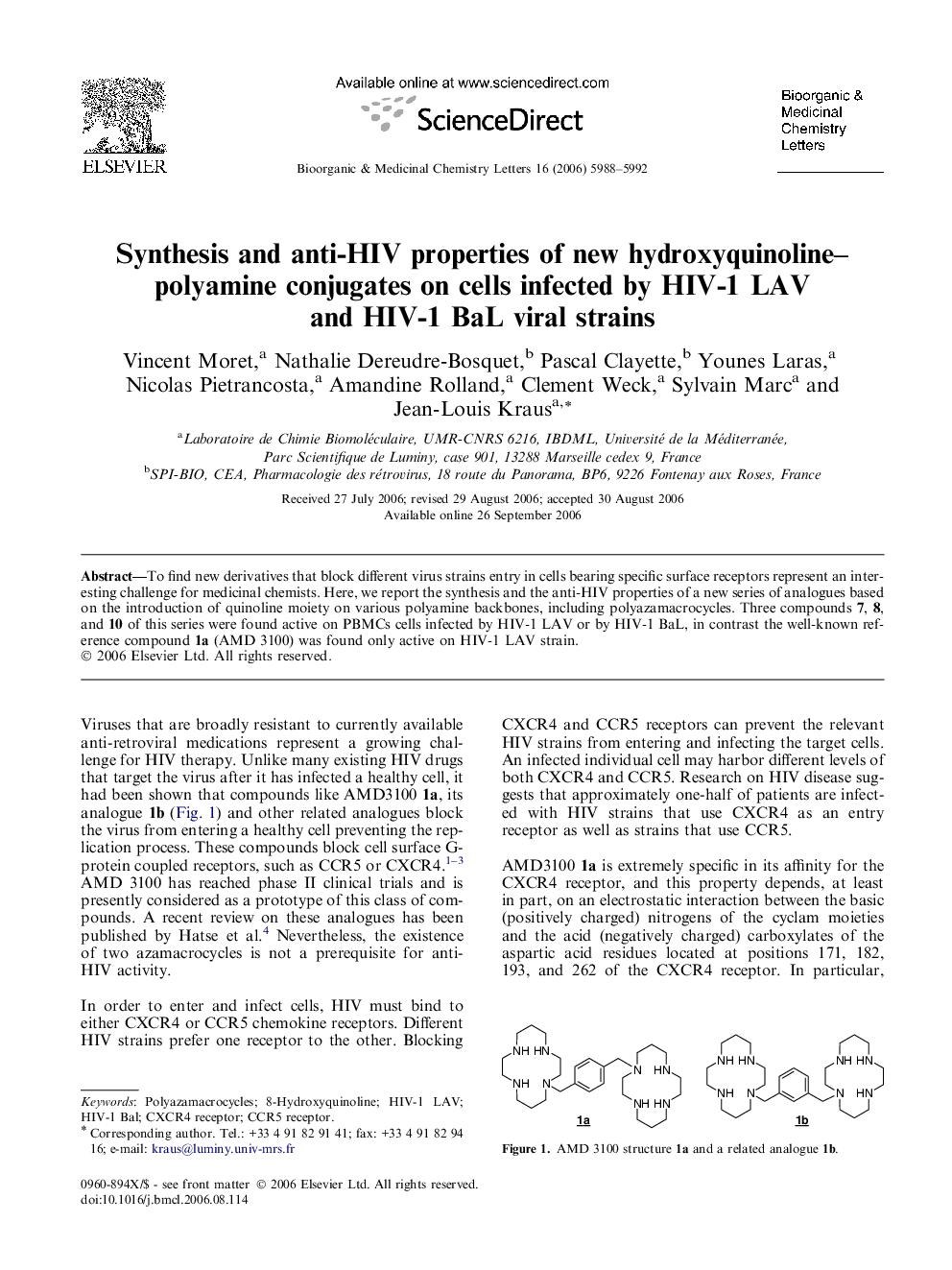
To find new derivatives that block different virus strains entry in cells bearing specific surface receptors represent an interesting challenge for medicinal chemists. Here, we report the synthesis and the anti-HIV properties of a new series of analogues based on the introduction of quinoline moiety on various polyamine backbones, including polyazamacrocycles. Three compounds 7, 8, and 10 of this series were found active on PBMCs cells infected by HIV-1 LAV or by HIV-1 BaL, in contrast the well-known reference compound 1a (AMD 3100) was found only active on HIV-1 LAV strain.
Graphical abstractGeneral structure of the new synthesized hydroxyquinoline–polyamine conjugates monocyclam and bicyclam–hydroxyquinoline conjugates 7, 8, and 10, respectively, found to be potent against HIV-1 LAV and HIV-1 BaL strains.Figure optionsDownload full-size imageDownload as PowerPoint slide