| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367318 | Bioorganic & Medicinal Chemistry Letters | 2006 | 4 Pages |
Abstract
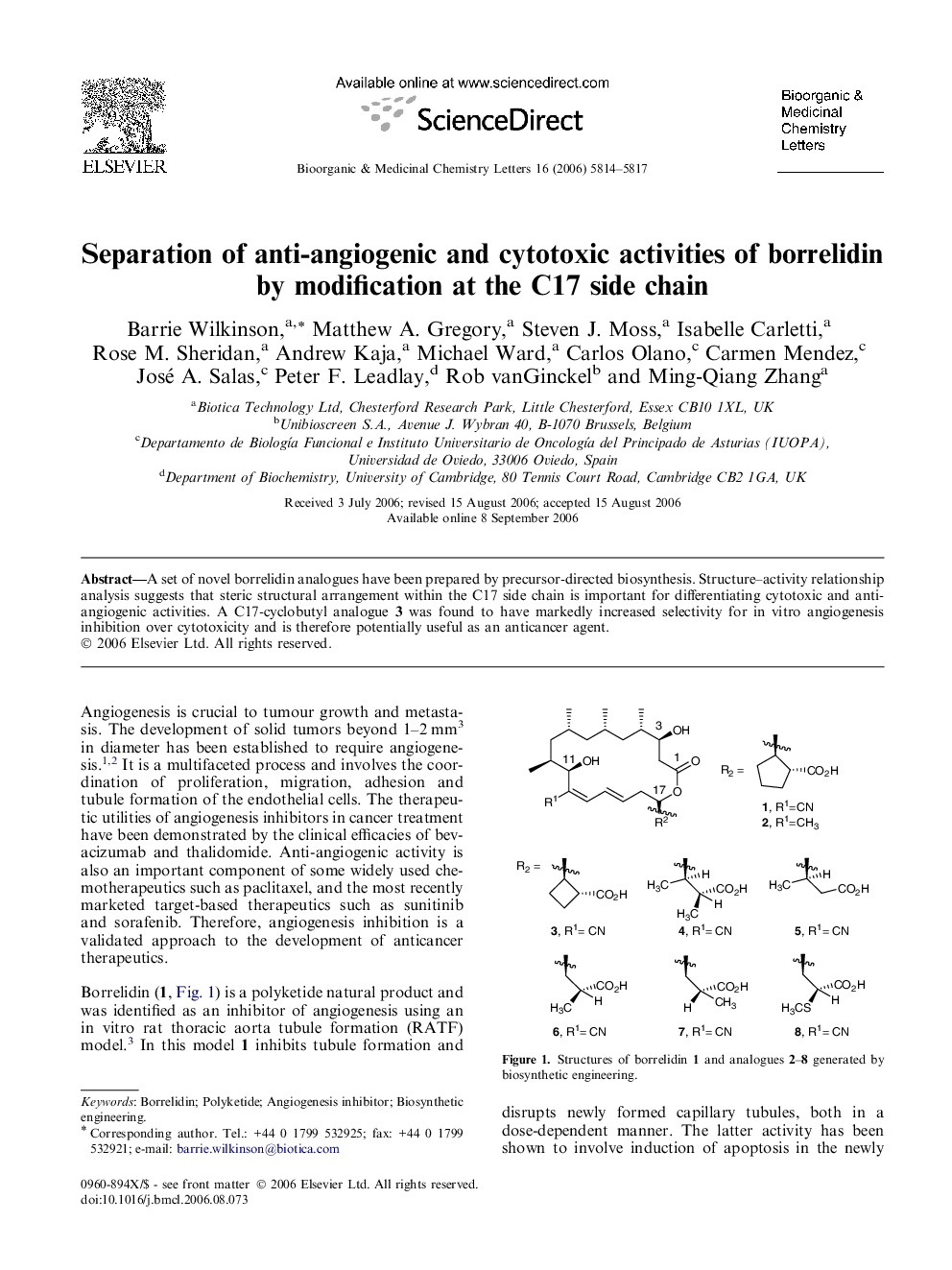
A set of novel borrelidin analogues have been prepared by precursor-directed biosynthesis. Structure–activity relationship analysis suggests that steric structural arrangement within the C17 side chain is important for differentiating cytotoxic and anti-angiogenic activities. A C17-cyclobutyl analogue 3 was found to have markedly increased selectivity for in vitro angiogenesis inhibition over cytotoxicity and is therefore potentially useful as an anticancer agent.
Graphical abstractAn SAR analysis of borrelidin analogues is presented.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Barrie Wilkinson, Matthew A. Gregory, Steven J. Moss, Isabelle Carletti, Rose M. Sheridan, Andrew Kaja, Michael Ward, Carlos Olano, Carmen Mendez, José A. Salas, Peter F. Leadlay, Rob vanGinckel, Ming-Qiang Zhang,