| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1367393 | Bioorganic & Medicinal Chemistry Letters | 2006 | 5 Pages |
Abstract
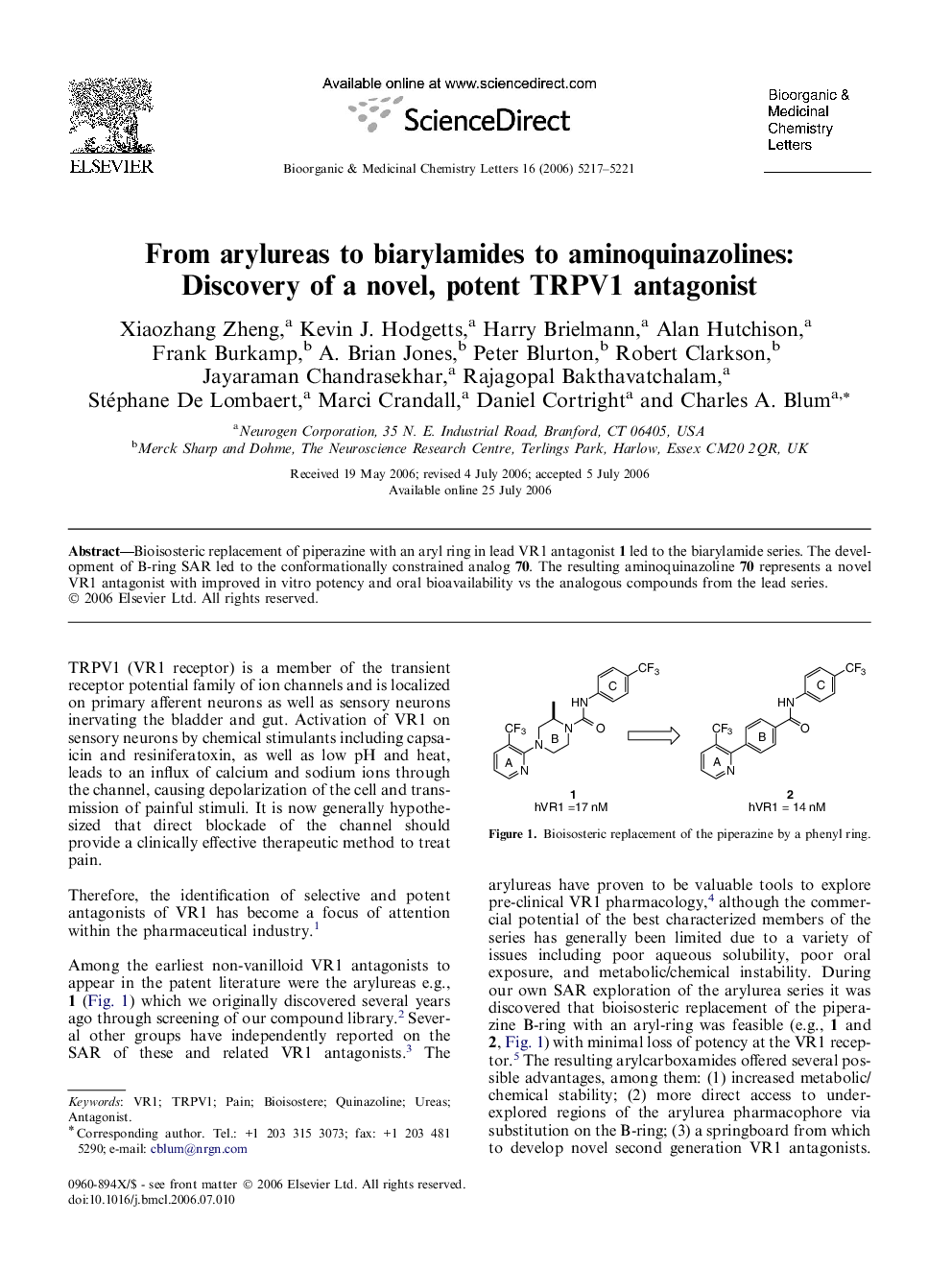
Bioisosteric replacement of piperazine with an aryl ring in lead VR1 antagonist 1 led to the biarylamide series. The development of B-ring SAR led to the conformationally constrained analog 70. The resulting aminoquinazoline 70 represents a novel VR1 antagonist with improved in vitro potency and oral bioavailability vs the analogous compounds from the lead series.
Graphical abstractA novel VR1 antagonist template (4-aminoquinazoline) exhibits improved in vitro potency and oral bioavailability relative to the corresponding urea or carboxamide compounds.Figure optionsDownload full-size imageDownload as PowerPoint slide
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Xiaozhang Zheng, Kevin J. Hodgetts, Harry Brielmann, Alan Hutchison, Frank Burkamp, A. Brian Jones, Peter Blurton, Robert Clarkson, Jayaraman Chandrasekhar, Rajagopal Bakthavatchalam, Stéphane De Lombaert, Marci Crandall, Daniel Cortright,